Fluidity of Poly (ε-Caprolactone)-Based Material Induces Epithelial-to-Mesenchymal Transition
Abstract
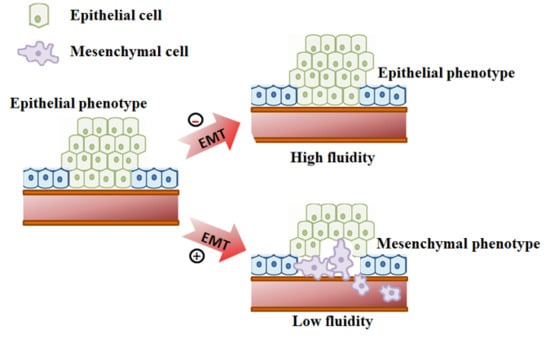
:1. Introduction
2. Results
2.1. Characterization of Crosslinked P(CL-co-DLLA) and Non-Crosslinked P(CL-co-DLLA) Substrates.
2.2. Thickness Based Effect of Non-crosslinked P(CL-co-DLLA) Substrates on Cell Spreading
2.3. Effect of Non-Crosslinked P (CL-co-DLLA) Substrates on E-Cadherin and Vimentin Expression
2.4. Effect of Substrate Fluidity on TGF-β Expression
2.5. Effect of Non-Crosslinked P(CL-co-DLLA) Substrate on Cell Adhesion
2.6. Effect of Non-crosslinked P(CL-co-DLLA) Substrate on Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Preparation of Non-Crosslinked P (CL-co-DLLA) Substrates
4.2. Preparation of Crosslinked P(CL-co-DLLA) Substrates
4.3. Cell Culture
4.4. Immunostaining and Confocal Microscopy
4.5. Cell Proliferation and Adhesion Assay
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| EMT | Epithelial to mesenchymal transition |
| ECM | Extracellular matrix |
| TGF α | Tumor necrosis factor α |
| NF-κB | Nuclear factor kappa B |
| IL-8 | Interleukin-8 |
| TGF-β | Transforming growth factor-β |
| PCL | poly(ε-caprolactone) |
| Mw | Molecular weight |
| Mn | Molecular number |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| FAK | Focal adhesion kinase |
| MET | Mesenchymal to epithelial transition |
| EndMT | Endothelial to mesenchymal transition |
| PAAm | Poly(acrylamide) |
| PDL | Poly-D-lysine |
| PEG | Poly(ethylene glycol) |
| EPC | Esophageal epithelial cells |
| PCL | Poly (ε-caprolactone) |
| DLLA | D,L,lactide |
| SEM | Scanning electron microscope |
| MEM | Minimum essential medium eagle’s |
| NEAA | Non-essential amino acid |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| BSA | Bovine serum albumin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
References
- Woods, D.F.; Bryant, P.J. Apical junctions and cell signalling in epithelia. J. Cell Sci. 1993, 17, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koohestani, F.; Braundmeier, A.G.; Mahdian, A.; Seo, J.; Bi, J.; Nowak, R.A. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells. PLoS ONE 2013, 11, e75844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daub, J.T.; Merks, R.M. A cell-based model of extracellular-matrix-guided endothelial cell migration during angiogenesis. Bull. Math. Biol. 2013, 75, 1377–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Klos, M.; Wilson, G.F.; Herman, A.M.; Lian, X.; Raval, K.K.; Barron, M.R.; Hou, L.; Soerens, A.G.; Yu, J.; et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: The matrix sandwich method. Circ. Res. 2012, 111, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Díaz, V.M.; Vinas-Castells, R.; de Herreros, A.G. Regulation of the protein stability of EMT transcription factors. Cell. Adh. Migr. 2014, 8, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, J.W. Membrane proteins involved in epithelial-mesenchymal transition and tumor invasion: Studies on TMPRSS4 and TM4SF5. Genomics Inform. 2014, 12, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Fang, Y.; Li, Z.; Chen, Z.; Xiang, J. Role of cellular cytoskeleton in epithelial-mesenchymal transition process during cancer progression. Biomed. Rep. 2015, 3, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.K.; Lee, K.; Radisky, D.C.; Nelson, C.M. Extracellular matrix proteins regulate epithelial-mesenchymal transition in mammary epithelial cells. Differentiation 2013, 86, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Bras, G.F.L.; Taubenslag, K.J.; Andl, C.D. The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell. Adh. Migr. 2012, 6, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abba, M.L.; Patil, N.; Leupold, J.H.; Allgayer, H. MicroRNA regulation of epithelial to mesenchymal transition. J. Clin. Med. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Mihalko, E.P.; Brown, A.C. Material strategies for modulating epithelial to mesenchymal transitions. ACS Biomater. Sci. Eng. 2018, 4, 1149–1161. [Google Scholar]
- Zhou, M.; Ma, H.; Lin, H.; Qin, J. Induction of epithelial-to-mesenchymal transition in proximal tubular epithelial cells on microfluidic devices. Biomaterials 2014, 35, 1390–1401. [Google Scholar] [CrossRef]
- Mano, S.S.; Uto, K.; Ebara, M. Material-induced senescence (MIS): Fluidity induces senescent type cell death of lung cancer cells via insulin-like growth factor binding protein 5. Theranostics 2017, 7, 4658–4670. [Google Scholar] [CrossRef] [Green Version]
- Jean-Léon, M.; Heisenberg, C. Three functions of cadherins in cell adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar]
- Shu, J.; Wang, L.; Han, F.; Chen, Y.; Wang, S.; Luo, F. BTBD7 downregulates E-cadherin and promotes epithelial-mesenchymal transition in lung cancer. Biol. Med. Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Chernoivanenkoa, I.S.; Minina, A.A.; MininbRussian, A.A. Role of vimentin in cell migration. J. Dev. Biol. 2013, 44, 144–157. [Google Scholar] [CrossRef]
- Bogachek, M.V.; De Andrade, J.P.; Weigel, R.J. Regulation of epithelial- mesenchymal transition through SUMOylation of transcription factors. Cancer Res. 2015, 75, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Uto, K.; Yamamoto, K.; Hirase, S.; Aoyagi, T. Temperature-responsive cross-linked poly(ε-caprolactone) membrane that functions near body temperature. J. Control. Release 2006, 110, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Uto, K.; Idota, N.; Hoffman, J.M.; Aoyagi, T. Shape-memory surface with dynamically tunable nano-geometry activated by body heat. Adv. Mater. 2012, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Ebara, M.; Aoyagi, T. Temperature-responsive poly(ε-caprolactone) cell culture platform with dynamically tunable nano-roughness and elasticity for control of myoblast morphology. Int. J. Mol. Sci. 2014, 15, 1511–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uto, K.; Mano, S.S.; Ebara, M.; Aoyagi, T. Substrate fluidity regulates cell adhesion and morphology on poly(ε-caprolactone)-based materials. ACS Biomater. Sci. Eng. 2016, 2, 446–453. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell. Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Wendt, M.K.; Allington, T.M.; Schiemann, W.P. Mechanisms of epithelial-mesenchymal transition by TGF-β. Future Oncol. 2009, 5, 1145–1168. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.A.; Aakre, M.E.; Gorska, A.E.; Price, J.O.; Eltom, S.E.; Pietenpol, J.A.; Moses, H.L. Induction by transforming growth factor-β1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004, 6, R215–R231. [Google Scholar] [CrossRef] [Green Version]
- Cicchini, C.; Laudadio, I.; Citarella, F.; Corazzari, M.; Steindler, C.; Conigliaro, A. TGF beta-induced EMT requires focal adhesion kinase (FAK) signalling. Exp. Cell Res. 2008, 314, 143–152. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Budd, E.; Mobasheri, A.; Mozafari, M. Biomaterials for regenerative medicine: Historical perspectives and current trends. Adv. Exp. Med. Biol. 2018, 1119, 1–19. [Google Scholar]
- Nagano, M.; Hoshino, D.; Koshikawa, N.; Akizawa, T.; Seiki, M. Turnover of focal adhesion and cancer cell migration. Int. J. Cell Biol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, M.J.; Phillips, S.R.; Shah, D.S.H.; Athey, D.; Lakey, J.H.; Przyborski, S.A. Enhanced cell attachment using a novel cell culture surface presenting functional domains from extracellular matrix proteins. Cytotechnology 2008, 56, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.S.; Teoh, S.H. Effect of stiffness of polycaprolactone (PCL) membrane on cell proliferation. Mater. Sci. Eng. C 2007, 27, 304–308. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [Green Version]
- Wingate, K.; Floren, M.; Tan, Y.; Tseng, P.O.; Tan, W. synergism of matrix stiffness and vascular endothelial growth factor on mesenchymal stem cells for vascular endothelial regeneration. Tissue Eng. Part A 2014, 20, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, N. Review of cellular mechanotransduction. J. Phys. D Appl. Phys. 2017, 50, 233002. [Google Scholar] [CrossRef]
- Hirt, U.A.; Waizenegger, I.C.; Schweifer, N.; Haslinger, C.; Gerlach, D.; Braunger, J.; Weyer-Czernilofsky, U.; Stadtmuller, H.; Sapountzis, I.; Bader, G.; et al. Efficacy of the highly selective focal adhesion kinase inhibitor BI 853520 in adenocarcinoma xenograft models is linked to a mesenchymal tumor phenotype. Oncogenesis 2018, 7. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, H.; Wang, L.; Ren, K.; Martins, M.C.; Barbosa, M.A.; Jian. J. Effect of polyelectrolyte film stiffness on endothelial cells during endothelial-to-mesenchymal transition. Biomacromolecules 2015, 16, 3584–3593. [Google Scholar] [CrossRef]
- Dhawan, U.; Sue, M.W.; Lan, K.C.; Buddhakosai, W.; Huang, P.H.; Chen, Y.C.; Chen, P.C.; Chen, W.L. Nanochip-induced epithelial-to-mesenchymal transition: Impact of physical microenvironment on cancer metastasis. ACS Appl. Mater. Interfaces 2018, 10, 11474–11485. [Google Scholar] [CrossRef]
- Nematbakhsh, Y.; Pang, K.T.; Lim, C.T. Correlating the viscoelasticity of breast cancer cells with their malignancy. Converg. Sci. Phys. Oncol. 2017, 3, 034003. [Google Scholar] [CrossRef]
- Ravikrishnan, A.; Ozdemir, T.; Bah, M.; Baskercille, K.A.; Shah, S.I.; Rajasekaran, A.K.; Jia, X. Regulation of epithelial-to-mesenchymal transition using biomimetic fibrous scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 17915–17926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Kokada, K.; Sasaki, N.; Chang, A.C.; Yamaguchi, K.; Nakanishi, J. Photoactivatable hydrogel interfaces for resolving the interplay of chemical, mechanical, and geometrical regulation of collective cell migration. Langmuir 2018, 35, 7459–7468. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hada, M.; Saha, J.; Sridharan, D.M.; Pluth, J.M.; Cucinotta, F.A. Protons sensitize epithelial cells to mesenchymal transition. PLoS ONE 2012, 7, e412492012. [Google Scholar]
- Son, H.; Moon, A. Epithelial-mesenchymal transition and cell invasion. Toxicol. Res. 2010, 26, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, H.; Allen, J.T.; Mason, R.M.; Kamimura, T.; Zhang, Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir. Res. 2005, 6, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Yu, L.; Hébert, M.C.; Zhang, Y.E. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 2002, 21, 3749–3759. [Google Scholar] [CrossRef] [Green Version]
- Barker, T.H.; Dysart, M.M.; Brown, M.A.; Douglas, A.M.; Fiore, V.F.; Russell, A.G. Synergistic effects of particulate matter and substrate stiffness on epithelial-to-mesenchymal transition. Health Eff. Inst. Res. Rep. 2014, 182, 3–41. [Google Scholar]







| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | CCCCCACCACACTGAATCTC | GCCCCTCCCCTCTTCAAG |
| E-cadherin | AACGCATTGCCACATACACTC | GACCTCCATCACAGAGGTTCC |
| vimentin | TCAATGTTAAGATGGCCCTTG | TGAGTGGGTATCAACCAGAGG |
| TGF-β | CCCAGCATCTGCAAAGCTC | GTCAATGTACAGCTGCCGCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mano, S.S.; Uto, K.; Ebara, M. Fluidity of Poly (ε-Caprolactone)-Based Material Induces Epithelial-to-Mesenchymal Transition. Int. J. Mol. Sci. 2020, 21, 1757. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051757
Mano SS, Uto K, Ebara M. Fluidity of Poly (ε-Caprolactone)-Based Material Induces Epithelial-to-Mesenchymal Transition. International Journal of Molecular Sciences. 2020; 21(5):1757. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051757
Chicago/Turabian StyleMano, Sharmy Saimon, Koichiro Uto, and Mitsuhiro Ebara. 2020. "Fluidity of Poly (ε-Caprolactone)-Based Material Induces Epithelial-to-Mesenchymal Transition" International Journal of Molecular Sciences 21, no. 5: 1757. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051757