Apical Sodium-Dependent Bile Acid Cotransporter, A Novel Transporter of Indocyanine Green, and Its Application in Drug Screening
Abstract
:1. Introduction
2. Results
2.1. Confirmation of ASBT and NTCP Tranduction
2.2. ICG Intake through ASBT
2.2.1. Confirmation of ICG Intake through ASBT
2.2.2. Pharmacokinetics of ICG Intake through ASBT
2.3. ICG Transportability between ASBT and NTCP
2.4. FITC Intake through ASBT
2.5. Combination of ICG with ASBT for in Vivo Cell Tracking
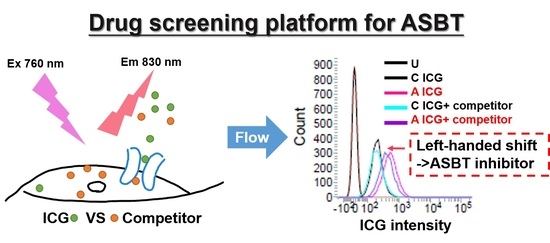
2.6. Combination of ICG with ASBT for Drug–drug Interaction
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Preparation
4.2. In Vitro ICG Intake
4.3. Pharmacokinetics of ICG
4.4. The Animal Experiment of ICG Intake
4.5. Drug-Screening Platform
4.6. In Vitro Intake of FITC
4.7. Cell Viability
4.8. Western Blotting
4.9. Immunofluorescence and Immunohistochemistry
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASBT | Apical sodium-dependent bile acid cotransporter |
| FITC | Fluorescein 5(6)-isothiocyanate |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GFP | Green fluorescent protein |
| NTCP | Sodium taurocholate cotransporting polypeptide |
| IVIS | In vivo imaging system |
| ICG | Indocyanine green |
| OATP1B3 | Organic-anion-transporting polypeptide 1B3 |
| TA | Taurocholate |
| SLC10 | Solute carrier 10 |
References
- Slijepcevic, D.; van de Graaf, S.F.J. Bile Acid Uptake Transporters as Targets for Therapy. Dig. Dis. 2017, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kramer, W.; Stengelin, S.; Baringhaus, K.H.; Enhsen, A.; Heuer, H.; Becker, W.; Corsiero, D.; Girbig, F.; Noll, R.; Weyland, C. Substrate specificity of the ileal and the hepatic Na+/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J. Lipid Res. 1999, 40, 1604–1617. [Google Scholar] [PubMed]
- Da Silva, T.C.; Polli, J.E.; Swaan, P.W. The solute carrier family 10 (SLC10): Beyond bile acid transport. Mol. Asp. Med. 2013, 34, 252–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Ekins, S.; Raufman, J.-P.P.; Polli, J.E. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol. Pharm. 2009, 6, 1591–1603. [Google Scholar] [CrossRef] [Green Version]
- Kramer, W.; Glombik, H. Bile acid reabsorption inhibitors (BARI): Novel hypolipidemic drugs. Curr. Med. Chem. 2006, 13, 997–1016. [Google Scholar] [CrossRef]
- Telford, D.E.; Edwards, J.Y.; Lipson, S.M.; Sutherland, B.; Barrett, P.H.R.; Burnett, J.R.; Krul, E.S.; Keller, B.T.; Huff, M.W. Inhibition of both the apical sodium-dependent bile acid transporter and HMG-CoA reductase markedly enhances the clearance of LDL apoB. J. Lipid Res. 2003, 44, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Boni, L.; David, G.; Mangano, A.; Dionigi, G.; Rausei, S.; Spampatti, S.; Cassinotti, E.; Fingerhut, A. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015, 29, 2046–2055. [Google Scholar] [CrossRef] [Green Version]
- James, N.S.; Chen, Y.; Joshi, P.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowsk, L.; Pandey, R.K. Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: Part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Guo, J.; Du, C.; Shan, L.; Zhu, H.; Xue, B.; Qian, Z.; Achilefu, S.; Gu, Y. Comparison of near-infrared fluorescent deoxyglucose probes with different dyes for tumor diagnosis in vivo. Contrast Media Mol. Imaging 2012, 7, 289–301. [Google Scholar] [CrossRef]
- Yu, S.; Cheng, B.; Yao, T.; Xu, C.; Nguyen, K.T.; Hong, Y.; Yuan, B. New generation ICG-based contrast agents for ultrasound-switchable fluorescence imaging. Sci. Rep. 2016, 6, 35942. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Sakaguchi, T.; Hiraide, T.; Morita, Y.; Suzuki, A.; Baba, S.; Setou, M.; Konno, H. Expression of indocyanine green-related transporters in hepatocellular carcinoma. J. Surg. Res. 2015, 193, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Semenenko, I.; Portnoy, E.; Aboukaoud, M.; Guzy, S.; Shmuel, M.; Itzhak, G.; Eyal, S. Evaluation of near infrared dyes as markers of P-glycoprotein activity in tumors. Front. Pharm. 2016, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- De Graaf, W.; Häusler, S.; Heger, M.; van Ginhoven, T.M.; van Cappellen, G.; Bennink, R.J.; Kullak-Ublick, G.A.; Hesselmann, R.; van Gulik, T.M.; Stieger, B. Transporters involved in the hepatic uptake of 99mTc-mebrofenin and indocyanine green. J. Hepatol. 2011, 54, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-R.; Liu, H.-M.; Lu, C.-W.; Shen, W.-H.; Lin, I.-J.; Liao, L.-W.; Huang, Y.-Y.; Shieh, M.-J.; Hsiao, J.-K. Organic anion-transporting polypeptide 1B3 as a dual reporter gene for fluorescence and magnetic resonance imaging. Faseb J. 2018, 32, 1705–1715. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-R.; Huang, Y.-Y.; Hsiao, J.-K. Role of Sodium Taurocholate Cotransporting Polypeptide as a New Reporter and Drug-Screening Platform: Implications for Preventing Hepatitis B Virus Infections. Mol. Imaging Biol. 2019. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-R.; Huang, Y.-Y.; Hsiao, J.-K. Use of Indocyanine Green (ICG), a Medical Near Infrared Dye, for Enhanced Fluorescent Imaging—Comparison of Organic Anion Transporting Polypeptide 1B3 (OATP1B3) and Sodium-Taurocholate Cotransporting Polypeptide (NTCP) Reporter Genes. Molecules 2019, 24, 2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, A.Q.; Balasubramaniyan, N.; Chen, H.; Shahid, M.; Suchy, F.J. Identification of functionally relevant residues of the rat ileal apical sodium-dependent bile acid cotransporter. J. Biol. Chem. 2006, 281, 16410–16418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthusamy, S.; Malhotra, P.; Hosameddin, M.; Dudeja, A.K.; Borthakur, S.; Saksena, S.; Gill, R.K.; Dudeja, P.K.; Alrefai, W.A. N-glycosylation is essential for ileal ASBT function and protection against proteases. Am. J. Physiol.-Cell Physiol. 2015, 308, C964–C971. [Google Scholar] [CrossRef] [Green Version]
- Hagenbuch, B.; Dawson, P. The sodium bile salt cotransport family SLC10. Pflug. Arch. Eur. J. Physiol. 2004, 447, 566–570. [Google Scholar] [CrossRef]
- Salic, K.; Kleemann, R.; Wilkins-Port, C.; McNulty, J.; Verschuren, L.; Palmer, M. Apical sodium-dependent bile acid transporter inhibition with volixibat improves metabolic aspects and components of nonalcoholic steatohepatitis in Ldlr-/-.Leiden mice. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Donkers, J.M.; Roscam Abbing, R.L.P.; van de Graaf, S.F.J. Developments in bile salt based therapies: A critical overview. Biochem. Pharm. 2019, 161, 1–13. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Pham, L.; Tietz, P.; Marinelli, R.A.; DeGroen, P.C.; Levine, S.; Dawson, P.A.; LaRusso, N.F. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J. Clin. Invest. 1997, 100, 2714–2721. [Google Scholar] [CrossRef]
- Pascolo, L.; Cupelli, F.; Anelli, P.L.; Lorusso, V.; Visigalli, M.; Uggeri, F.; Tiribelli, C. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem. Biophys. Res. Commun. 1999, 257, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, M.; Keiser, M.; Oswald, S.; Kuhn, J.; Jia, J.; Grube, M.; Kroemer, H.K.; Siegmund, W.; Weitschies, W. Hepatic Uptake of the Magnetic Resonance Imaging Contrast Agent Gd-EOB-DTPA: Role of Human Organic Anion Transporters. Drug Metab. Dispos. 2010, 38, 1024–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, W.J.; Lapsley, M.; Day, A.P.; Ayling, R.M. Clinical Biochemistry: Metabolic and Clinical Aspects, 3th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; ISBN 9780702054785. [Google Scholar]
- Balakrishnan, A.; Polli, J.E. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): A potential prodrug target. Mol. Pharm. 2006, 3, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, P.A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharm. 2011, 201, 169–203. [Google Scholar]
- Viale, M.; Cordazzo, C.; De Totero, D.; Budriesi, R.; Rosano, C.; Leoni, A.; Ioan, P.; Aiello, C.; Croce, M.; Andreani, A.; et al. Inhibition of MDR1 activity and induction of apoptosis by analogues of nifedipine and diltiazem: An in vitro analysis. Invest. New Drugs 2011, 29, 98–109. [Google Scholar] [CrossRef]
- Portnoy, E.; Gurina, M.; Magdassi, S.; Eyal, S. Evaluation of the near infrared compound indocyanine green as a probe substrate of P-glycoprotein. Mol. Pharm. 2012, 9, 3595–3601. [Google Scholar] [CrossRef]
- Butcher, E.C.; Weissman, I.L. Direct fluorescent labeling of cells with fluorescein or rhodamine isothiocyanate. I. Technical aspects. J. Immunol. Methods 1980, 37, 97–108. [Google Scholar] [CrossRef]
- Tiscornia, G.; Singer, O.; Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 2006, 1, 241–245. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-R.; Hsiao, J.-K. Apical Sodium-Dependent Bile Acid Cotransporter, A Novel Transporter of Indocyanine Green, and Its Application in Drug Screening. Int. J. Mol. Sci. 2020, 21, 2202. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21062202
Wu M-R, Hsiao J-K. Apical Sodium-Dependent Bile Acid Cotransporter, A Novel Transporter of Indocyanine Green, and Its Application in Drug Screening. International Journal of Molecular Sciences. 2020; 21(6):2202. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21062202
Chicago/Turabian StyleWu, Menq-Rong, and Jong-Kai Hsiao. 2020. "Apical Sodium-Dependent Bile Acid Cotransporter, A Novel Transporter of Indocyanine Green, and Its Application in Drug Screening" International Journal of Molecular Sciences 21, no. 6: 2202. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21062202