Tailored PCL Scaffolds as Skin Substitutes Using Sacrificial PVP Fibers and Collagen/Chitosan Blends
Abstract
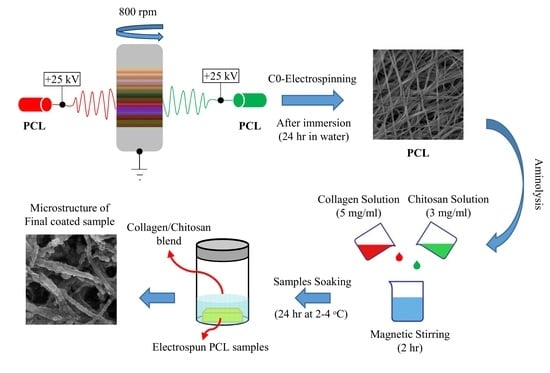
:1. Introduction
2. Results
2.1. Microstructural Observations
2.2. Mechanical Properties
2.3. In Vitro Evaluations
2.3.1. Cell Attachment
2.3.2. MTT Assay
2.3.3. Cell Infiltration
2.3.4. Antibacterial Assay
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Electrospinning
4.2.1. Solution Preparation
4.2.2. Fiber Formation via Electrospinning
4.3. Surface Modification of Electrospun Samples
4.3.1. Preparation of the Biopolymers Solution
4.3.2. Aminolysis and Coating
4.4. Characterization
4.4.1. Morphological Observations
4.4.2. Porosimetry
4.4.3. X-ray Photoelectron Spectroscopy (XPS)
4.4.4. Tensile Test
4.4.5. Cell Attachment
4.4.6. MTT Assay
4.4.7. Cell Infiltration
4.4.8. Antibacterial Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chae, T.; Ko, F. Electrospun nanofibrous tissue scaffolds. Electrospun Nanofibers 2016, 521–550. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, Z.; Stylios, G.K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.L.; Gomes, S.; Henriques, C.; Borges, J.P.; Silva, J.C. Electrospinning polycaprolactone dissolved in glacial acetic acid: Fiber production, nonwoven characterization, and In Vitro evaluation. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Cruz, Y.; Muñoz, E.; Gomez-Pachón, E.Y.; Morales-Corona, J.; Olayo-Lortia, J.; Olayo, R.; Olayo-Valles, R. Electrospun PCL-protein scaffolds coated by pyrrole plasma polymerization. J. Biomater. Sci. Polym. Ed. 2019, 30, 832–845. [Google Scholar] [CrossRef]
- Safaeijavan, R.; Soleimani, M.; Divsalar, A.; Eidi, A. Biological behavior study of gelatin coated PCL nanofiberous electrospun scaffolds using fibroblasts. J. Paramed. Sci. 2014, 5, 67–73. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, S.; Ai, J.; Azami, M.; Verdi, J.; Atlasi, M.A.; Shirian, S.; Samadikuchaksaraei, A. Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: Preliminary physico-chemical studies for skin substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Croisier, F.; Sibret, P.; Dupont-Gillain, C.C.; Genet, M.J.; Detrembleur, C.; Jérôme, C. Chitosan-coated electrospun nanofibers with antibacterial activity. J. Mater. Chem. B 2015, 3, 3508–3517. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Ramalingam, R.; Dhand, C.; Lakshminarayanan, R.; Sadiq, A.; Gandhimathi, C.; Ramakrishna, S.; Bay, H.B.; Venugopal, R.J.; Srinivasan, K.D. Biocompatible Aloe vera and Tetracycline Hydrochloride Loaded Hybrid Nanofibrous Scaffolds for Skin Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5174. [Google Scholar] [CrossRef] [Green Version]
- Jeznach, O.; Kolbuk, D.; Sajkiewicz, P. Aminolysis of Various Aliphatic Polyesters in a Form of Nanofibers and Films. Polymers 2019, 11, 1669. [Google Scholar] [CrossRef] [Green Version]
- Permyakova, S.E.; Kiryukhantsev-Korneev, V.P.; Gudz, Y.K.; Konopatsky, S.A.; Polčak, J.; Zhitnyak, Y.I.; Gloushankova, A.N.; Shtansky, V.D.; Manakhov, M.A. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [Green Version]
- Yew, H.C.; Azari, P.; Choi, R.J.; Muhamad, F.; Pingguan-Murphy, B. Electrospun Polycaprolactone Nanofibers as a Reaction Membrane for Lateral Flow Assay. Polymers 2018, 10, 1387. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shi, L.; Zhang, X.; Liu, K.; Ullah, I.; Cheng, P. Electrospinning of polycaprolactone nanofibers using H2O as benign additive in polycaprolactone/glacial acetic acid solution. J. Appl. Polym. Sci. 2018, 135, 45578. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface Modification of Polycaprolactone Membrane via Aminolysis and Biomacromolecule Immobilization for Promoting Cytocompatibility of Human Endothelial Cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E.; Staroszczyk, H.; Gottfried, K.; Kołodziejska, I.; Wojtasz-Pająk, A. Antimicrobial properties of chitosan solutions, chitosan films and gelatin-chitosan films. Polimery/Polymers 2015, 60, 735–741. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Ameer, M.J.; Pr, K.A.; Kasoju, N. Strategies to Tune Electrospun Scaffold Porosity for Effective Cell Response in Tissue Engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Mao, Z.; Shi, H.; Gao, C. In-depth study on aminolysis of poly(ɛ-caprolactone): Back to the fundamentals. Sci. China Chem. 2012, 55, 2419–2427. [Google Scholar] [CrossRef]
- Gentile, P.; McColgan-Bannon, K.; Gianone, N.C.; Sefat, F.; Dalgarno, K.; Ferreira, A.M. Biosynthetic PCL-graft-Collagen Bulk Material for Tissue Engineering Applications. Materials 2017, 10, 693. [Google Scholar] [CrossRef] [Green Version]
- Zander, N.E.; Orlicki, J.A.; Rawlett, A.M.; Beebe, T.P. Electrospun polycaprolactone scaffolds with tailored porosity using two approaches for enhanced cellular infiltration. J. Mater. Sci. Mater. Med. 2013, 24, 179–187. [Google Scholar] [CrossRef]
- Denchai, A.; Tartarini, D.; Mele, E. Cellular Response to Surface Morphology: Electrospinning and Computational Modeling. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.T.; Johnson, J.; Lannutti, J. Media-based effects on the hydrolytic degradation and crystallization of electrospun synthetic-biologic blends. J. Mater. Sci. Mater. Med. 2014, 25, 297–309. [Google Scholar] [CrossRef]
- Prasad, T.; Shabeena, E.A.; Vinod, D.; Kumary, T.V.; Anil Kumar, P.R. Characterization and in vitro evaluation of electrospun chitosan/polycaprolactone blend fibrous mat for skin tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 28. [Google Scholar] [CrossRef]
- Nhi, T.T.; Khon, H.C.; Hoai, N.T.T.; Bao, B.C.; Quyen, T.N.; Van Toi, V.; Hiep, N.T. Fabrication of electrospun polycaprolactone coated withchitosan-silver nanoparticles membranes for wound dressing applications. J. Mater. Sci. Mater. Med. 2016, 27, 156. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, Z.; Zheng, L.; Song, R.; Zhao, Y. Fabrication and Characterization of Electrospun Polycaprolactone Blended with Chitosan-Gelatin Complex Nanofibrous Mats. J. Nanomater. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; He, C.; Wang, H. Intermolecular interactions in electrospun collagen–chitosan complex nanofibers. Carbohydr. Polym. 2008, 72, 410–418. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Kennedy, C.J.; Wess, T.J. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Bolgen, N.; Salgado, A.J.; Gomes, M.E.; Piskin, E.; Reis, R.L. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005, 16, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Shabani, I.; Haddadi-Asl, V.; Seyedjafari, E.; Soleimani, M. Cellular infiltration on nanofibrous scaffolds using a modified electrospinning technique. Biochem. Biophys. Res. Commun. 2012, 423, 50–54. [Google Scholar] [CrossRef]
- Chen, L.; Al-Shawk, A.; Rea, C.; Mazeh, H.; Wu, X.; Chen, W.; Li, Y.; Song, W.; Markel, D.C.; Ren, W. Preparation of electrospun nanofibers with desired microstructures using a programmed three-dimensional (3D) nanofiber collector. Mater. Sci. Eng. C 2020, 106, 110188. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the Pore Size of Electrospun Scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Jeong, S.I.; Burns, N.A.; Bonino, C.A.; Kwon, I.K.; Khan, S.A.; Alsberg, E. Improved cell infiltration of highly porous 3D nanofibrous scaffolds formed by combined fiber–fiber charge repulsions and ultra-sonication. J. Mater. Chem. B 2014, 2, 8116–8122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, C.M.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Sadeghi-Avalshahr, A. Preparation of PVA/Chitosan samples by electrospinning and film casting methods and evaluating the effect of surface morphology on their antibacterial behavior. Mater. Res. Express 2019, 7, 015401. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, M.A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Erdem, B.; Kurt, M.; Okur, S. Morphological analysis of the antibacterial action of chitosan on gram-negative bacteria using atomic force microscopy. Curr. Opin. Biotechnol. 2013, 24, S83. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: A review. Sci. Total Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef]












| Microorganism | Contact time (h) | Sample | (CFU/mL) | Antibacterial Activity (%) |
|---|---|---|---|---|
| E. coli (ATCC25922) | 1 | A | 1.5 × 106 | no antibacterial activity |
| B | 1.5 × 106 | no antibacterial activity | ||
| 24 | A | 1.25 × 106 | 16.66 | |
| B | 4.7 × 105 | 68.66 | ||
| S. aureus (ATCC6538) | 1 | A | 1.5 × 106 | no antibacterial activity |
| B | 1.5 × 106 | no antibacterial activity | ||
| 24 | A | 1.3 × 106 | 13.33 | |
| B | 9.5 × 105 | 36.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi-avalshahr, A.R.; Nokhasteh, S.; Molavi, A.M.; Mohammad-pour, N.; Sadeghi, M. Tailored PCL Scaffolds as Skin Substitutes Using Sacrificial PVP Fibers and Collagen/Chitosan Blends. Int. J. Mol. Sci. 2020, 21, 2311. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072311
Sadeghi-avalshahr AR, Nokhasteh S, Molavi AM, Mohammad-pour N, Sadeghi M. Tailored PCL Scaffolds as Skin Substitutes Using Sacrificial PVP Fibers and Collagen/Chitosan Blends. International Journal of Molecular Sciences. 2020; 21(7):2311. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072311
Chicago/Turabian StyleSadeghi-avalshahr, Ali Reza, Samira Nokhasteh, Amir Mahdi Molavi, Najmeh Mohammad-pour, and Mohammad Sadeghi. 2020. "Tailored PCL Scaffolds as Skin Substitutes Using Sacrificial PVP Fibers and Collagen/Chitosan Blends" International Journal of Molecular Sciences 21, no. 7: 2311. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072311