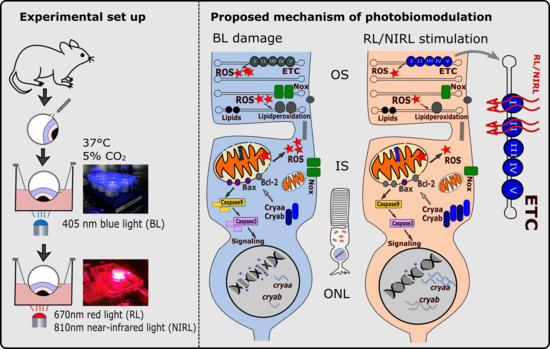
Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration
Abstract
:1. Introduction
2. Results
2.1. Decreased Mitochondria-Induced Apoptosis upon RL/NIRL Exposure
2.2. Increased Respiration upon RL/NIRL Exposure
2.3. Decreased ROS Levels after RL/NIRL Exposure
2.4. RL/NIRL Regulated the Expression of Genes Associated with Protective Functions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Organ Culture and Irradiation with Light
4.3. Intracellular ROS Production
4.4. Measurement of ATP Content
4.5. Histochemical Reactions for ETC I and II Activity
4.6. Oxygraphic Measurements
4.7. Laser Microdissection of Photoreceptors
4.8. RNA Isolation
4.9. Sequencing and Bioinformatic Analysis
4.10. In-situ Hybridization
4.11. Further Analyses
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| BL | Blue light |
| C | Control |
| CCO | Cytochrome c oxidase |
| DCFDA | 2′,7′–dichlorofluorescin diacetate |
| DEG | Differentially expressed genes |
| ETC | Electron transport chain |
| GCL | Ganglion cell layer |
| INL | Inner nuclear layer |
| IS | Inner segments |
| LCM | Laser capture microdissection |
| NIRL | Near-infrared light |
| ONL | Outer nuclear layer |
| ONL | Outer nuclear layer |
| OS | Outer segments |
| OXPHOS | Oxidative phosphorylation |
| PBM | Photobiomodulation |
| RL | Red light |
| ROI | Region of interest |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| SEM | Standard error of the mean |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling |
References
- Desmet, K.D.; Paz, D.A.; Corry, J.J.; Eells, J.T.; Wong-Riley, M.T.; Henry, M.M.; Buchmann, E.V.; Connelly, M.P.; Dovi, J.V.; Liang, H.L.; et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed. Laser Surg. 2006, 24, 121–128. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Turrioni, A.P.S.; Bagnato, V.S.; Hebling, J.A. In Vitro Wound Healing Improvement by Low-Level Laser Therapy Application in Cultured Gingival Fibroblasts. Int. J. Dent. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-level laser therapy for wound healing: Mechanism and efficacy. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2005, 31, 334–340. [Google Scholar] [CrossRef]
- Giacci, M.K.; Wheeler, L.; Lovett, S.; Dishington, E.; Majda, B.; Bartlett, C.A.; Thornton, E.; Harford-Wright, E.; Leonard, A.; Vink, R.; et al. Differential Effects of 670 and 830 nm Red near Infrared Irradiation Therapy: A Comparative Study of Optic Nerve Injury, Retinal Degeneration, Traumatic Brain and Spinal Cord Injury. PLoS ONE 2014, 9, e104565. [Google Scholar] [CrossRef] [Green Version]
- Beirne, K.; Rozanowska, M.; Votruba, M. Red Light Treatment in an Axotomy Model of Neurodegeneration. Photochem. Photobiol. 2016, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong-Riley, M.T.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Whelan, H.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef] [Green Version]
- Lapchak, P.A. Transcranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseases. Expert Rev. Med. Devices 2013, 9, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, K.R.; Waynant, R.W.; Ilev, I.K.; Wu, X.; Barna, L.; Smith, K.; Heckert, R.; Gerst, H.; Anders, J.J. Light Promotes Regeneration and Functional Recovery and Alters the Immune Response After Spinal Cord Injury. Lasers Surg. Med. 2005, 36, 171–185. [Google Scholar] [CrossRef]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Karu, T.I. Multiple Roles of Cytochrome c Oxidase in Mammalian Cells Under Action of Red and IR-A Radiation. Iubmb Life 2010, 62, 607–610. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B Biol. 2005, 81, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.Z.; Fernando, N.; Natoli, R.; Madigan, M.; Valter, K. 670nm light treatment following retinal injury modulates Müller cell gliosis: Evidence from in vivo and in vitro stress models. Exp. Eye Res. 2018, 169, 1–12. [Google Scholar] [CrossRef]
- Osborne, N.N.; Nez-lvarez, C.; del Olmo-Aguado, S.; Merrayo-Lloves, J. Visual light effects on mitochondria: The potential implications in relation to glaucoma. Mitochondrion 2017, 36, 29–35. [Google Scholar] [CrossRef]
- del Olmo-Aguado, S.; Nez-lvarez, C.; Osborne, N.N. Red light of the visual spectrum attenuates cell death in culture and retinal ganglion cell death in situ. Acta Ophthalmol. 2016, 94, e481–e491. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Du, Y.; Lee, C.A.; Talahalli, R.; Eells, J.T.; Kern, T.S. Low-Intensity Far-Red Light Inhibits Early Lesions That Contribute to Diabetic Retinopathy: In Vivo and In Vitro. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef] [Green Version]
- Fuma, S.; Murase, H.; Kuse, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. Photobiomodulation with 670 nm light increased phagocytosis in human retinal pigment epithelial cells. Mol. Vis. 2015, 21, 883–892. [Google Scholar]
- Rutar, M.; Natoli, R.; Albarracin, R.; Valter, K.; Provis, J. 670-nm light treatment reduces complement propagation following retinal degeneration. J. Neuroinflammation 2012, 9, 257. [Google Scholar] [CrossRef] [Green Version]
- Albarracin, R.; Natoli, R.; Rutar, M.; Valter, K.; Provis, J. 670 nm light mitigates oxygen-induced degeneration in C57BL/6J mouse retina. BMC Neurosci. 2013, 14, 125. [Google Scholar] [CrossRef] [Green Version]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation Protects the Retina from Light-Induced Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef] [Green Version]
- Begum, R.; Powner, M.B.; Hudson, N.; Hogg, C.; Jeffery, G. Treatment with 670 nm Light Up Regulates Cytochrome C Oxidase Expression and Reduces Inflammation in an Age-Related Macular Degeneration Model. PLoS ONE 2013, 8, e57828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkinopoulos, I. 670 nm LED ameliorates inflammation in the CFH-/- mouse neural retina. J. Photochem. Photobiol. B Biol. 2013, 122, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Valter, K.; Barbosa, M.; Dahlstrom, J.; Rutar, M.; Kent, A.; Provis, J. 670nm Photobiomodulation as a Novel Protection against Retinopathy of Prematurity: Evidence from Oxygen Induced Retinopathy Models. PLoS ONE 2013, 8, e72135. [Google Scholar] [CrossRef] [PubMed]
- Gkotsi, D.; Begum, R.; Salt, T.; Lascaratos, G.; Hogg, C.; Chau, K.Y.; Schapira, A.H.V.; Jeffery, G. Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Exp. Eye Res. 2014, 122, 50–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivapathasuntharam, C.; Sivaprasad, S.; Hogg, C.; Jeffery, G. Aging retinal function is improved by near infrared light (670 nm) that is associated with corrected mitochondrial decline. Neurobiol. Aging 2017, 52, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulos, I.; Colman, A.; Hogg, C.; Heckenlively, J.; Jeffery, G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol. Aging 2013, 34, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Ivandic, B.T.; Ivandic, T. Low-Level Laser Therapy Improves Vision in Patients with Age-Related Macular Degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kaynezhad, P.; Tachtsidis, I.; Jeffery, G. Optical monitoring of retinal respiration in real time: 670 nm light increases the redox state of mitochondria. Exp. Eye Res. 2016, 152, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehlecke, C.; Schumann, U.; Ader, M.; Knels, L.; Funk, R.H.W. Influence of blue light on photoreceptors in a live retinal explant system. Mol. Vis. 2011, 17, 876–884. [Google Scholar]
- Roehlecke, C.; Schumann, U.; Ader, M.; Brunssen, C.; Bramke, S.; Morawietz, H.; Funk, R.H.W. Stress Reaction in Outer Segments of Photoreceptors after Blue Light Irradiation. PLoS ONE 2013, 8, e71570. [Google Scholar] [CrossRef]
- Schäfer, P.; Karl, M.O. Prospective purification and characterization of Müller glia in the mouse retina regeneration assay. Glia 2017, 65, 828–847. [Google Scholar] [CrossRef] [PubMed]
- Retinal cell death dependent reactive proliferative gliosis in the mouse retina. Sci. Rep. 2017, 7, 1–16. [CrossRef] [Green Version]
- Krigel, A.; Berdugo, M.; Picard, E.; Levy-Boukris, R.; Jaadane, I.; Jonet, L.; Dernigoghossian, M.; Andrieu-Soler, C.; Torriglia, A.; Behar-Cohen, F. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience 2016, 339, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzia, D.; Garbarino, G.; Caicci, F.; Manni, L.; Candiani, S.; Ravera, S.; Morelli, A.; Traverso, C.E.; Panfoli, I. Functional expression of electron transport chain complexes in mouse rod outer segments. Biochimie 2014, 102, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Novel roles for alpha-crystallins in retinal function and disease. Prog. Retin. Eye Res. 2012, 31, 576–604. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.W.; Liu, J.P.; Xiang, H.; Li, D.W.C. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-Xs to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004, 11, 512–526. [Google Scholar] [CrossRef]
- Gu, R.; Tang, W.; Lei, B.; Ding, X.; Jiang, C.; Xu, G. Glucocorticoid-induced leucine zipper protects the retina from light-induced retinal degeneration by inducing Bcl-xL in rats. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3656–3668. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.L.; Whelan, H.T.; Eells, J.T.; Meng, H.; Buchmann, E.; Lerch-Gaggl, A.; Wong-Riley, M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience 2006, 139, 639–649. [Google Scholar] [CrossRef]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.E.; et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef] [Green Version]
- Ghafourifar, P.; Klein, S.D.; Schucht, O.; Schenk, U.; Pruschy, M.; Rocha, S.; Richter, C. Ceramide induces cytochrome c release from isolated mitochondria. J. Biol. Chem. 1999, 274, 6080–6084. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xing, D.; Zhu, D.; Chen, Q. Low-Power Laser Irradiation Inhibiting Aβ25-35 -induced PC12 Cell Apoptosis via PKC Activation. Cell. Physiol. Biochem. 2008, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Shefer, G.; Partridge, T.a.; Heslop, L.; Gross, J.G.; Oron, U.; Halevy, O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J. Cell Sci. 2002, 115, 1461–1469. [Google Scholar] [PubMed]
- Panfoli, I.; Calzia, D.; Bianchini, P.; Ravera, S.; Diaspro, A.; Candiano, G.; Bachi, A.; Monticone, M.; Aluigi, M.G.; Barabino, S.; et al. Evidence for aerobic metabolism in retinal rod outer segment disks. Int. J. Biochem. Cell Biol. 2009, 41, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.H.; Wider, J.M.; Lee, I.; Reynolds, C.A.; Liu, J.; Lepore, B.; Tousignant, R.; Bukowski, M.J.; Johnston, H.; Fite, A.; et al. Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Park, J.K.; Hwang, S.-G.; Kim, W.-J.; Liu, Z.-G.; Kang, S.W.; Um, H.-D. Nuclear and cytoplasmic p53 suppress cell invasion by inhibiting respiratory Complex-I activity via Bcl-2 family proteins. Oncotarget 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of Oxidative Metabolism and Electron Chain Enzymes in Rat Liver Mitochondria. Photochem. Photobiol. 2008, 66, 866–871. [Google Scholar] [CrossRef]
- Lima, P.L.V.; Pereira, C.V.; Nissanka, N.; Arguello, T.; Gavini, G.; Maranduba, C.M.d.C.; Diaz, F.; Moraes, C.T. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. J. Photochem. Photobiol. B Biol. 2019, 194, 71–75. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. Biochim. Et Biophys. Acta Bioenerg. 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [Green Version]
- Calzia, D.; Barabino, S.; Bianchini, P.; Garbarino, G.; Oneto, M.; Caicci, F.; Diaspro, A.; Tacchetti, C.; Manni, L.; Candiani, S.; et al. New findings in ATP supply in rod outer segments: Insights for retinopathies. Biol. Cell 2013, 105, 345–358. [Google Scholar] [CrossRef]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzia, D.; Panfoli, I.; Heinig, N.; Schumann, U.; Ader, M.; Traverso, C.E.; Funk, R.H.W.; Roehlecke, C. Impairment of extramitochondrial oxidative phosphorylation in mouse rod outer segments by blue light irradiation. Biochimie 2016, 125, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tafur, J.; Mills, P.J. Low-Intensity Light Therapy: Exploring the Role of Redox Mechanisms. Photomed. Laser Surg. 2008, 26, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.C.H.; Arany, P.R.; Huang, Y.-Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-Level Laser Therapy Activates NF-kB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Voehringer, D.W.; Meyn, R.E. Redox Aspects of Bcl-2 Function. Antioxid. Redox Signal. 2000, 2, 537–550. [Google Scholar] [CrossRef]
- Hockenbery, D.M.; Oltvai, Z.N.; Yin, X.M.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Functions in an Antioxidant Pathway to Prevent Apoptosis. Cell 1993, 75, 241–251. [Google Scholar] [CrossRef]
- Hall, H.; Ma, J.; Shekhar, S.; Leon-Salas, W.D.; Weake, V.M. Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors. BMC Neurosci. 2018, 19, 43. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, L.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel Top. Quantum Electron. 2016, 22, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, A.S.; Singh, B.N.; Rao, K.S.; Rao, C.M. AlphaB-crystallin, a small heat shock protein, modulates NF-ΚB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim. Et Biophys. Acta Mol. Cell Res. 2011, 1813, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Noh, S.J.; Jeong, W.J.; Rho, J.H.; Shin, D.M.; Ahn, H.B.; Park, W.C.; Rho, S.H.; Soung, Y.H.; Kim, T.H.; Park, B.S.; et al. Sensitization of RPE Cells by lphaB-Crystallin siRNA to SAHA-Induced Stage 1 Apoptosis through Abolishing the Association of alphaB-Crystallin with HDAC1 in SC35 Speckles. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Yaung, J.; Jin, M.; Barron, E.; Spee, C.; Wawrousek, E.F.; Kannan, R.; Hinton, D.R. Alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol. Vis. 2007, 13, 566–577. [Google Scholar] [PubMed]
- Kannan, R.; Ouyang, B.; Wawrousek, E.; Kaplowitz, N.; Andley, U.P. Regulation of GSH in alphaA-Expressing Human lens Epithelial Cell Lines and in alphaA Knockout Mouse Lenses. Investig. Ophthalmol. Vis. Sci. 2001, 42, 409–416. [Google Scholar]
- Sakaguchi, H.; Miyagi, M.; Darrow, R.M.; Crabb, J.S.; Hollyfield, J.G.; Organisciak, D.T.; Crabb, J.W. Intense light exposure changes the crystallin content in retina. Exp. Eye Res. 2003, 76, 131–133. [Google Scholar] [CrossRef]
- Organisciak, D.; Darrow, R.; Barsalou, L.; Rapp, C.; McDonald, B.; Wong, P. Light Induced and Circadian Effects on Retinal Photoreceptor Cell Crystallins. Photochem. Photobiol. 2011, 87, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, M.K.; Saraswathy, S.; Parikh, J.G.; Rao, N.A. The Role of TLR4 Activation in Photoreceptor Mitochondrial Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5824–5835. [Google Scholar] [CrossRef]
- Rao, N.A.; Saraswathy, S.; Wu, G.S.; Katselis, G.S.; Wawrousek, E.F.; Bhat, S. Elevated Retina-Specific Expression of the Small Heat Shock Protein, alphaA-crystallin, Is Associated with Photoreceptor Protection in Experimental Uveitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Maeda, A.; Ohguro, H.; Maeda, T.; Nakagawa, T.; Kuroki, Y. Low Expression of alphaA-Crystallins and Rhodopsin Kinase of Photoreceptors in Retinal Dystrophy Rat. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2788–2794. [Google Scholar]
- Kase, S.; He, S.; Sonoda, S.; Kitamura, M.; Spee, C.; Wawrousek, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. AlphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 2010, 115, 3398–3406. [Google Scholar] [CrossRef]
- Munemasa, Y.; Kwong, J.M.K.; Caprioli, J.; Piri, N. The Role of alphaA- and alphaB-Crystallins in the Survival of Retinal Ganglion Cells after Optic Nerve Axotomy. Investig. Opthalmology Vis. Sci. 2009, 50, 3869. [Google Scholar] [CrossRef] [Green Version]
- Burns, M.E.; Mendez, A.; Chen, C.-K.; Almuete, A.; Quillinan, N.; Simon, M.I.; Baylor, D.A.; Chen, J. Deactivation of Phosphorylated and Nonphosphorylated Rhodopsin by Arrestin Splice Variants. J. Neurosci. 2006, 26, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Aicardi, G.; Solaini, G. Effects of niridazole and 5-nitroimidazoles on heart mitochondrial respiration. Biochem. Pharmacol. 1982, 31, 3703–3705. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinig, N.; Schumann, U.; Calzia, D.; Panfoli, I.; Ader, M.; Schmidt, M.H.H.; Funk, R.H.W.; Roehlecke, C. Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration. Int. J. Mol. Sci. 2020, 21, 2370. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072370
Heinig N, Schumann U, Calzia D, Panfoli I, Ader M, Schmidt MHH, Funk RHW, Roehlecke C. Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration. International Journal of Molecular Sciences. 2020; 21(7):2370. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072370
Chicago/Turabian StyleHeinig, Nora, Ulrike Schumann, Daniela Calzia, Isabella Panfoli, Marius Ader, Mirko H. H. Schmidt, Richard H. W. Funk, and Cora Roehlecke. 2020. "Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration" International Journal of Molecular Sciences 21, no. 7: 2370. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072370