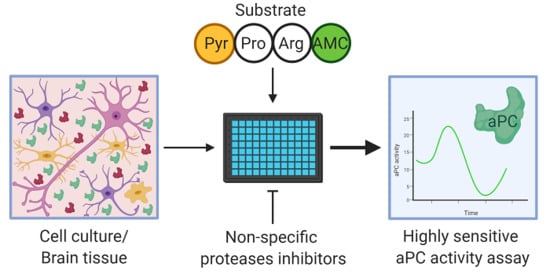
A Novel Highly Sensitive Method for Measuring Inflammatory Neural-Derived APC Activity in Glial Cell Lines, Mouse Brain and Human CSF
Abstract
:1. Background
2. Results
2.1. Assay Validation
2.2. Cell Cultures
2.3. Mice, Healthy Control, and Mild Traumatic Brain Injury (mTBI)
2.4. The Effect of Inflammation, LPS in Mice, and Human Inflammatory CSF
3. Discussion
4. Methods
4.1. Cell Culture
4.2. Animals
4.3. Mild Traumatic Brain Injury (mTBI) Induction
4.4. Mice Lipopolysaccharide (LPS) Treatment
4.5. Human Patients
4.6. APC Activity Assay
4.7. Thrombin, FXa, Plasma, and PC
4.8. Experimental Design
4.8.1. Thrombin/FXa/Plasma
4.8.2. Cells
4.8.3. Brain Tissue
4.9. Western Blot Analysis
4.9.1. Samples Preparation
4.9.2. Protein Detection
4.10. Statistics
Author Contributions
Funding
Acknowledgments
Disclosure
Conflicts of Interest
Abbreviations
| Activated protein C | APC |
| Thrombomodulin | TM |
| Mild traumatic brain injury | mTBI |
| Cerebrospinal fluid | CSF |
| Lipopolysaccharide | LPS |
| Endothelial protein C receptor | EPCR |
| Protease activator receptor 1 | PAR1 |
| Peripheral nervous system | PNS |
| Central nervous system | CNS |
| Normal pressure hydrocephalus | NPH |
| Phenylmethylsulfonyl fluoride | PMSF |
| Alpha-naphthylsulphonylglycyl-4-amidinophenylalanine piperidine | NAPAP |
References
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Kore, R.A.; Abraham, E.C. Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochem. Biophys. Res. Commun. 2014, 453, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellewell, S.; Semple, B.D.; Morganti-Kossmann, M.C. Therapies negating neuroinflammation after brain trauma. Brain Res. 2016, 1640, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Jaberi, N.; Avan, A.; Ryzhikov, M.; Keramati, M.R.; Parizadeh, M.R.; Hassanian, S.M. Role of thrombin in the pathogenesis of central nervous system inflammatory diseases. J. Cell. Physiol. 2017, 232, 482–485. [Google Scholar] [CrossRef]
- Duprez, D.A.; Neuhaus, J.; Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE 2012, 7, e44454. [Google Scholar] [CrossRef]
- McClintock, D.; Zhuo, H.; Wickersham, N.; Matthay, M.A.; Ware, L.B. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit. Care 2008, 12, R41. [Google Scholar] [CrossRef] [Green Version]
- Gould, T.W.; Dominguez, B.; de Winter, F.; Yeo, G.W.; Liu, P.; Sundararaman, B.; Stark, T.; Vu, A.; Degen, J.L.; Lin, W.; et al. Glial cells maintain synapses by inhibiting an activity-dependent retrograde protease signal. PLoS Genet. 2019, 15, e1007948. [Google Scholar] [CrossRef]
- Stein, E.S.; Itsekson-Hayosh, Z.; Aronovich, A.; Reisner, Y.; Bushi, D.; Pick, C.G.; Tanne, D.; Chapman, J.; Vlachos, A.; Maggio, N. Thrombin induces ischemic LTP (iLTP): Implications for synaptic plasticity in the acute phase of ischemic stroke. Sci. Rep. 2015, 5, 7912. [Google Scholar] [CrossRef] [Green Version]
- Ben Shimon, M.; Zeimer, T.; Shavit Stein, E.; Artan-Furman, A.; Harnof, S.; Chapman, J.; Eisenkraft, A.; Pick, C.G.; Maggio, N. Recovery from trauma induced amnesia correlates with normalization of thrombin activity in the mouse hippocampus. PLoS ONE 2017, 12, e0188524. [Google Scholar] [CrossRef] [Green Version]
- Esmon, C.T. The protein C pathway. Chest 2003, 124, 26S–32S. [Google Scholar] [CrossRef] [Green Version]
- Esmon, C.T. Targeting factor Xa and thrombin: Impact on coagulation and beyond. Thromb. Haemost. 2014, 111, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Festoff, B.W.; Citron, B.A. Thrombin and the Coag-Inflammatory Nexus in Neurotrauma, ALS, and Other Neurodegenerative Disorders. Front. Neurol. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C. Inflammation and the Activated Protein C Anticoagulant Pathway. Semin. Thromb. Hemost. 2006, 32, 049–060. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, M.; Fernández, J.; Lane, S.; Griffin, G.; Zlocovik, B. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J. Neurosci. 2008, 28, 12788–12797. [Google Scholar] [CrossRef] [Green Version]
- Mahajan-Thakur, S.; Böhm, A.; Jedlitschky, G.; Schrör, K.; Rauch, B.H. Sphingosine-1-Phosphate and Its Receptors: A Mutual Link between Blood Coagulation and Inflammation. Mediat. Inflamm. 2015, 2015, 831059. [Google Scholar] [CrossRef] [Green Version]
- Gera, O.; Shavit-Stein, E.; Bushi, D.; Harnof, S.; Shimon, M.B.; Weiss, R.; Golderman, V.; Dori, A.; Maggio, N.; Finegold, K.; et al. Thrombin and protein C pathway in peripheral nerve Schwann cells. Neuroscience 2016, 339, 587–598. [Google Scholar] [CrossRef]
- Maggio, N.; Itsekson, Z.; Ikenberg, B.; Strehl, A.; Vlachos, A.; Blatt, I.; Tanne, D.; Chapman, J. The anticoagulant activated protein C (aPC) promotes metaplasticity in the hippocampus through an EPCR-PAR1-S1P1 receptors dependent mechanism. Hippocampus 2014, 24, 1030–1038. [Google Scholar] [CrossRef]
- Asmat, A.; Ramzan, F. Venom Protein C Activators as Diagnostic Agents for Defects of Protein C System. Protein Pept. Lett. 2018, 25, 643–651. [Google Scholar] [CrossRef]
- Griffin, J.H.; Gruber, A. Direct detection of activated protein C in blood from human subjects. Blood 1992, 79, 2340–2348. [Google Scholar]
- Beilin, O.; Gurwitz, D.; Korczyn, A.D.; Chapman, J. Quantitative measurements of mouse brain thrombin-like and thrombin inhibition activities. Neuroreport 2001, 12, 2347–2351. [Google Scholar] [CrossRef]
- Bushi, D.; Chapman, J.; Katzav, A.; Shavit-Stein, E.; Molshatzki, N.; Maggio, N.; Tanne, D. Quantitative detection of thrombin activity in an ischemic stroke model. J. Mol. Neurosci. 2013, 51, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Bushi, D.; Stein, E.S.; Golderman, V.; Feingold, E.; Gera, O.; Chapman, J.; Tanne, D. A linear temporal increase in thrombin activity and loss of its receptor in mouse brain following ischemic stroke. Front. Neurol. 2017, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavit-Stein, E.; Sheinberg, E.; Golderman, V.; Sharabi, S.; Wohl, A.; Gofrit, S.G.; Zivli, Z.; Shelestovich, N.; Last, D.; Guez, D.; et al. A Novel Compound Targeting Protease Receptor 1 Activators for the Treatment of Glioblastoma. Front. Neurol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.; Burm, S.M.; Bajramovic, J.J. An overview of in vitro methods to study microglia. Front. Cell. Neurosci. 2018, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Galland, F.; Seady, M.; Taday, J.; Smaili, S.S.; Gonçalves, C.A.; Leite, M.C. Astrocyte culture models: Molecular and function characterization of primary culture, immortalized astrocytes and C6 glioma cells. Neurochem. Int. 2019, 131, 104538. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef]
- Oto, J.; Fernandez-Pardo, A.; Miralles, M.; Plana, E.; Espana, F.; Navarro, S.; Medina, P. Activated protein C assays: A review. Clin. Chim. Acta 2020, 502, 227–232. [Google Scholar] [CrossRef]
- Guo, H.; Liu, D.; Gelbard, H.; Cheng, T.; Insalaco, R.; Fernández, J.A.; Griffin, J.H.; Zlokovic, B.V. Activated Protein C Prevents Neuronal Apoptosis via Protease Activated Receptors 1 and 3. Neuron 2004, 41, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 577. [Google Scholar] [CrossRef] [Green Version]
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Z.; Yang, Q.; Wang, M.; Bell, R.D.; Wang, S.; Chow, N.; Davis, T.P.; Griffin, J.H.; Goldman, S.A.; et al. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J. Neurosci. 2013, 33, 6181–6190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2019, 25, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef] [Green Version]
- Shavit Stein, E.; Ben Shimon, M.; Artan Furman, A.; Golderman, V.; Chapman, J.; Maggio, N. Thrombin Inhibition Reduces the Expression of Brain Inflammation Markers upon Systemic LPS Treatment. Neural Plast. 2018, 2018, 7692182. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.A.; Xu, X.; Liu, D.; Zlokovic, B.V.; Griffin, J.H. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells Mol. Dis. 2003, 30, 271–276. [Google Scholar] [CrossRef]
- Das, U. HLA-DR expression, cytokines and bioactive lipids in sepsis. Arch. Med. Sci. 2014, 10, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, P.P.; Lee, H.; Kim, M. Activated protein C action in inflammation. Br. J. Haematol. 2010, 148, 817–833. [Google Scholar] [CrossRef] [Green Version]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golderman, V.; Gofrit, S.G.; Maggio, N.; Gera, O.; Gerasimov, A.; Laks, D.; Chapman, J.; Shavit-Stein, E. A Novel Highly Sensitive Method for Measuring Inflammatory Neural-Derived APC Activity in Glial Cell Lines, Mouse Brain and Human CSF. Int. J. Mol. Sci. 2020, 21, 2422. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072422
Golderman V, Gofrit SG, Maggio N, Gera O, Gerasimov A, Laks D, Chapman J, Shavit-Stein E. A Novel Highly Sensitive Method for Measuring Inflammatory Neural-Derived APC Activity in Glial Cell Lines, Mouse Brain and Human CSF. International Journal of Molecular Sciences. 2020; 21(7):2422. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072422
Chicago/Turabian StyleGolderman, Valery, Shany G. Gofrit, Nicola Maggio, Orna Gera, Alexandra Gerasimov, Dar Laks, Joab Chapman, and Efrat Shavit-Stein. 2020. "A Novel Highly Sensitive Method for Measuring Inflammatory Neural-Derived APC Activity in Glial Cell Lines, Mouse Brain and Human CSF" International Journal of Molecular Sciences 21, no. 7: 2422. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21072422