Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling
Abstract
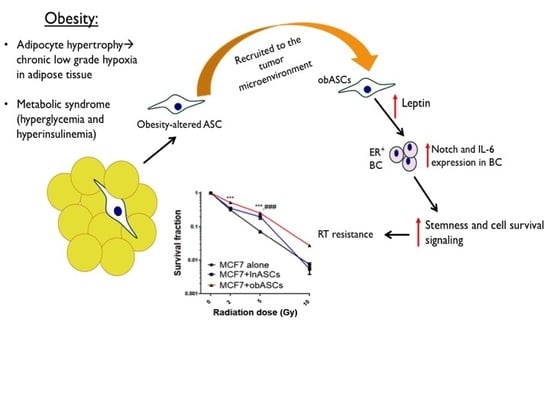
:1. Introduction
2. Results
2.1. Obesity-Altered ASCs Promote Radiation Resistance of ER+BCCs Lines
obASCs Promote Radiation Resistance through Decreased Oxidative Stress in ER+BCCs and Promoting a Higher Percentage of Cells in S-Phase
2.2. obASCs Produce Increased Leptin, which Promotes Radiation Resistance through IL-6 Upregulation
2.3. Leptin from obASCs Promotes a Cancer Stem-Like Phenotype through NOTCH Signaling
3. Discussion
4. Materials and Methods
4.1. Adipose Stem Cells
4.2. Cell Culture
4.3. Co-Culture Studies
4.4. Cell Cycle Analysis
4.5. Live Cell Imaging
4.6. Surviving Fraction
4.7. Xenograft Model
4.8. RT-qPCR analysis
4.9. Mammospheres
4.10. Flow Cytometry
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCs | Adipose stem cells |
| TME | Tumor microenvironment |
| obASCs | Obesity-altered ASCs |
| ER+BCC | Estrogen receptor positive breast cancer cells |
| RT | Radiotherapy |
| lnASCs | Lean ASCs |
| ER+ | Estrogen receptor positive |
| BC | Breast cancer |
| BCC | Breast cancer cells |
| TNBC | Triple negative breast cancer |
| Gy | Gray |
| SEM | Standard error of the mean |
| CCM | Complete culture media |
| shLep | Leptin shRNA |
| shCtrl | Control shRNA |
| BMI | Body mass index |
References
- Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html; http://www.breastcancer.org/symptoms/understand_bc/statistics; (accessed on 1 November 2018).
- About Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer.html (accessed on 1 November 2018).
- Goodwin, A.; Parker, S.; Ghersi, D.; Wilcken, N. Post-operative radiotherapy for ductal carcinoma in situ of the breast--a systematic review of the randomised trials. Breast 2009, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [PubMed]
- Wapnir, I.L.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Anderson, S.J.; Julian, T.B.; Land, S.R.; Margolese, R.G.; Swain, S.M.; Costantino, J.P.; et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in nsabp b-17 and b-24 randomized clinical trials for dcis. J. Natl. Cancer Inst. 2011, 103, 478–488. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.F.; Mazzarello, S.; Caudrelier, J.M.; Lima, M.A.G.; Hutton, B.; Sienkiewicz, M.; Stober, C.; Fernandes, R.; Ibrahim, M.F.K.; Vandermeer, L.; et al. Optimal sequence of adjuvant endocrine and radiation therapy in early-stage breast cancer - a systematic review. Cancer Treat. Rev. 2018, 69, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F.; Committee, E.G. Primary breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), 8–30. [Google Scholar] [CrossRef] [PubMed]
- McCall, N.S.; Simone, B.A.; Mehta, M.; Zhan, T.; Ko, K.; Nowak-Choi, K.; Rese, A.; Venkataraman, C.; Andrews, D.W.; Anne, P.R.; et al. Onco-metabolism: Defining the prognostic significance of obesity and diabetes in women with brain metastases from breast cancer. Breast Cancer Res. Treat. 2018, 172, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.J.; Wang, J.; Ahearn, T.U.; Brown, S.B.; Zhang, X.; Ambrosone, C.B.; de Andrade, V.P.; Brufsky, A.M.; Couch, F.J.; King, T.A.; et al. Molecular mechanisms linking high body mass index to breast cancer etiology in post-menopausal breast tumor and tumor-adjacent tissues. Breast Cancer Res. Treat. 2018, 173, 667–677. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Ogunsina, K.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res. Treat. 2018, 174, 209–218. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Platz, E.A.; Ligibel, J.A.; Blair, C.K.; Courneya, K.S.; Meyerhardt, J.A.; Ganz, P.A.; Rock, C.L.; Schmitz, K.H.; Wadden, T.; et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1244–1259. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.C.M.; Oh, S.Y.T.; Noh, O.K.; Kang, S.H.; Jang, H.; Cho, H.S. Risk factors for regional recurrence in node-negative breast cancer: Implications of regional nodal irradiation. Int. J. Radiat. Oncol. 2017, 99, E23. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sabol, R.A.; Bowles, A.C.; Cote, A.; Wise, R.; Pashos, N.; Bunnell, B.A. Therapeutic potential of adipose stem cells. Adv. Exp. Med. Biol. 2018, 1–11. [Google Scholar]
- Strong, A.L.B.A.; Wise, R.M.; Morand, J.P.; Dutreil, M.F.; GImble, J.M.; Bunnell, B.A. Human adipose stromal/stem cells from obese donors show reduced efficacy in halting disease progression in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Stem Cells 2016, 34, 614–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badimon, L.; Cubedo, J. Adipose tissue depots and inflammation: Effects on plasticity and resident mesenchymal stem cell function. Cardiovasc Res. 2017, 113, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- van Harmelen, V.; Skurk, T.; Rohrig, K.; Lee, Y.M.; Halbleib, M.; Aprath-Husmann, I.; Hauner, H. Effect of bmi and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Strong, A.L.S.T.; Rhodes, L.V.; Semon, J.A.; Zhang, X.; SHi, Z.; Zhang, S.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity associated alteration in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013, R102. [Google Scholar] [CrossRef] [Green Version]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Strong, A.L.O.J.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; Dutreil, M.F.; Zhang, S.; Gimble, J.M.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 112. [Google Scholar] [CrossRef] [Green Version]
- Sabol, R.A.; Beighley, A.; Giacomelli, P.; Wise, R.M.; Harrison, M.A.A.; O’Donnnell, B.A.; Sullivan, B.N.; Lampenfeld, J.D.; Matossian, M.D.; Bratton, M.R.; et al. Obesity-altered adipose stem cells promote er(+) breast cancer metastasis through estrogen independent pathways. Int. J. Mol. Sci. 2019, 20, 1419. [Google Scholar] [CrossRef] [Green Version]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. Us incidence of breast cancer subtypes defined by joint hormone receptor and her2 status. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [Green Version]
- Hafer, K.; Rivina, L.; Schiestl, R.H. Cell cycle dependence of ionizing radiation-induced DNA deletions and antioxidant radioprotection in saccharomyces cerevisiae. Radiat. Res. 2010, 173, 802–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, N.; Wang, X.; Wang, Y.; Iliakis, G. Loss of s-phase-dependent radioresistance in irs-1 cells exposed to x-rays. Mutat. Res. 1994, 314, 77–85. [Google Scholar] [CrossRef]
- Nicolay, N.H.; Carter, R.; Hatch, S.B.; Schultz, N.; Prevo, R.; McKenna, W.G.; Helleday, T.; Sharma, R.A. Homologous recombination mediates s-phase-dependent radioresistance in cells deficient in DNA polymerase eta. Carcinogenesis 2012, 33, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Yao, Q.; Liu, Y.; Du, S.; Liu, A.; Guo, Z.; Sun, A.; Ruan, J.; Chen, L.; Ye, C.; et al. Il-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int. J. Oncol. 2012, 40, 1171–1179. [Google Scholar]
- Li, H.J.; Reinhardt, F.; Herschman, H.R.; Weinberg, R.A. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin e2 signaling. Cancer Discov. 2012, 2, 840–855. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Shen, S.; Zhou, Y.; Mao, F.; Lin, Y.; Guan, J.; Xu, Y.; Zhang, S.; Liu, X.; Sun, Q. Notch1 is a poor prognostic factor for breast cancer and is associated with breast cancer stem cells. Onco Targets Ther. 2016, 9, 6865–6871. [Google Scholar] [CrossRef] [Green Version]
- Leon-Cabrera, S.; Solis-Lozano, L.; Suarez-Alvarez, K.; Gonzalez-Chavez, A.; Bejar, Y.L.; Robles-Diaz, G.; Escobedo, G. Hyperleptinemia is associated with parameters of low-grade systemic inflammation and metabolic dysfunction in obese human beings. Front. Integr. Neurosci. 2013, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Lagadec, C.; Vlashi, E.; Alhiyari, Y.; Phillips, T.M.; Bochkur Dratver, M.; Pajonk, F. Radiation-induced notch signaling in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Theys, J.; Yahyanejad, S.; Habets, R.; Span, P.; Dubois, L.; Paesmans, K.; Kattenbeld, B.; Cleutjens, J.; Groot, A.J.; Schuurbiers, O.C.J.; et al. High notch activity induces radiation resistance in non small cell lung cancer. Radiother. Oncol. 2013, 108, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Lanier, V.; Gillespie, C.; Leffers, M.; Daley-Brown, D.; Milner, J.; Lipsey, C.; Webb, N.; Anderson, L.M.; Newman, G.; Waltenberger, J.; et al. Leptin-induced transphosphorylation of vascular endothelial growth factor receptor increases notch and stimulates endothelial cell angiogenic transformation. Int. J. Biochem. Cell Biol. 2016, 79, 139–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, Y.; Kong, D.; Banerjee, S.; Ahmad, A.; Azmi, A.S.; Ali, S.; Abbruzzese, J.L.; Gallick, G.E.; Sarkar, F.H. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009, 69, 2400–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Gonzalez-Perez, R.R. Notch, il-1 and leptin crosstalk outcome (nilco) is critical for leptin-induced proliferation, migration and vegf/vegfr-2 expression in breast cancer. PLoS ONE 2011, 6, e21467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battle, M.; Gillespie, C.; Quarshie, A.; Lanier, V.; Harmon, T.; Wilson, K.; Torroella-Kouri, M.; Gonzalez-Perez, R.R. Obesity induced a leptin-notch signaling axis in breast cancer. Int. J. Cancer 2014, 134, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Newman, G.; Gonzalez-Perez, R.R. Leptin-cytokine crosstalk in breast cancer. Mol. Cell Endocrinol. 2014, 382, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.O.; Yang, X.; Duan, S.; Tsai, Y.; Strojny, L.R.; Keng, P.; Chen, Y. Il-6 promotes growth and epithelial-mesenchymal transition of cd133+ cells of non-small cell lung cancer. Oncotarget 2016, 7, 6626–6638. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.T.; Chen, M.F.; Chen, W.C.; Hsieh, C.C. The role of il-6 in the radiation response of prostate cancer. Radiat. Oncol. 2013, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- Eiro, N.; Gonzalez, L.; Gonzalez, L.O.; Fernandez-Garcia, B.; Lamelas, M.L.; Marin, L.; Gonzalez-Reyes, S.; del Casar, J.M.; Vizoso, F.J. Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PLoS ONE 2012, 7, e49047. [Google Scholar] [CrossRef] [Green Version]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived jagged1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Dong, J.; Wang, L.; Xia, Q.; Zhang, D.; Kim, H.; Yin, T.; Fan, S.; Shen, Q. Activation of stat3 and bcl-2 and reduction of reactive oxygen species (ros) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene 2018, 37, 5292–5304. [Google Scholar] [CrossRef]
- Munshi, A.; Hobbs, M.; Meyn, R.E. Clonogenic cell survival assay. Methods Mol. Med. 2005, 110, 21–28. [Google Scholar] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabol, R.A.; Villela, V.A.; Denys, A.; Freeman, B.T.; Hartono, A.B.; Wise, R.M.; Harrison, M.A.A.; Sandler, M.B.; Hossain, F.; Miele, L.; et al. Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling. Int. J. Mol. Sci. 2020, 21, 2722. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21082722
Sabol RA, Villela VA, Denys A, Freeman BT, Hartono AB, Wise RM, Harrison MAA, Sandler MB, Hossain F, Miele L, et al. Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling. International Journal of Molecular Sciences. 2020; 21(8):2722. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21082722
Chicago/Turabian StyleSabol, Rachel A., Vidal A. Villela, Alexandra Denys, Benjamin T. Freeman, Alifiani B. Hartono, Rachel M. Wise, Mark A. A. Harrison, Maxwell B. Sandler, Fokhrul Hossain, Lucio Miele, and et al. 2020. "Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling" International Journal of Molecular Sciences 21, no. 8: 2722. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21082722