Photo-Responsive Supramolecular Micelles for Controlled Drug Release and Improved Chemotherapy
Abstract
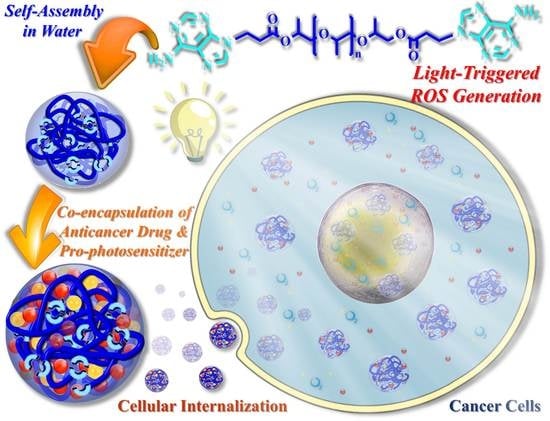
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Preparation of DOX/5-ALA-Loaded A-PPG Micelles
3.4. Evaluation of the Stability of DOX/5-ALA-Loaded Micelles
3.5. In Vitro DOX Release Assay
3.6. Cell Culture
3.7. In Vitro Cytotoxicity Studies
3.8. Intracellular ROS Assay
3.9. Analysis of Cellular Uptake by Confocal Laser Scanning Microscopy
3.10. Detection of DOX Fluorescence Intensity by Flow Cytometry
3.11. Flow Cytometric Analysis of Apoptosis by Annexin V/PI Double Staining
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhen, S.; Yi, X.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Drug delivery micelles with efficient near-infrared photosensitizer for combined image-guided photodynamic therapy and chemotherapy of drug-resistant cancer. Biomaterials 2019, 218, 119330. [Google Scholar] [PubMed]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. PEGylated hydrazided gold nanorods for pH-triggered chemo/photodynamic/photothermal triple therapy of breast cancer. Acta Biomater. 2018, 82, 171–183. [Google Scholar]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [PubMed] [Green Version]
- Holohan, C.; Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [PubMed]
- Wang, Y.; Yang, M.; Qian, J.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Sequentially self-assembled polysaccharide-based nanocomplexes for combined chemotherapy and photodynamic therapy of breast cancer. Carbohydr. Polym. 2019, 203, 203–213. [Google Scholar]
- Wang, T.; Wang, D.; Yu, H.; Wang, M.; Liu, J.; Feng, B.; Zhou, F.; Yin, Q.; Zhang, Z.; Huang, Y.; et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano 2016, 10, 3496–3508. [Google Scholar]
- Soenen, S.J.; Demeester, J.; De Smedt, S.C.; Braeckmans, K. Turning a frown upside down: Exploiting nanoparticle toxicity for anticancer therapy. Nano Today 2013, 8, 121–125. [Google Scholar]
- De Vera, A.A.; Reznik, S.E. Chapter 14—Combining PI3K/Akt/mTOR inhibition with chemotherapy. In Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Academic Press: Cambridge, MA, USA, 2019; pp. 229–242. [Google Scholar]
- Yokoi, K.; Tanei, T.; Godin, B.; van de Ven, A.L.; Hanibuchi, M.; Matsunoki, A.; Alexander, J.; Ferrari, M. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Lett. 2014, 345, 48–55. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control Release 2017, 248, 96–116. [Google Scholar]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control Release 2017, 258, 226–253. [Google Scholar] [PubMed]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [PubMed]
- Cheng, C.C.; Chang, F.C.; Kao, W.Y.; Hwang, S.M.; Liao, L.C.; Chang, Y.J.; Liang, M.C.; Chen, J.K.; Lee, D.J. Highly efficient drug delivery systems based on functional supramolecular polymers: In vitro evaluation. Acta Biomater. 2016, 33, 194–202. [Google Scholar]
- Alemayehu, Y.A.; Gebeyehu, B.T.; Cheng, C.C. Photosensitive supramolecular micelles with complementary hydrogen bonding motifs to improve the efficacy of cancer chemotherapy. Biomacromolecules 2019, 20, 4535–4545. [Google Scholar]
- Guo, Z.; Sui, J.; Ma, M.; Hu, J.; Sun, Y.; Yang, L.; Fan, Y.; Zhang, X. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J. Control Release 2020, 326, 350–364. [Google Scholar] [PubMed]
- Cheng, C.C.; Sun, Y.T.; Lee, A.W.; Huang, S.Y.; Fan, W.L.; Chiao, Y.H.; Tsai, H.C.; Lai, J.Y. Self-assembled supramolecular micelles with pH-responsive properties for more effective cancer chemotherapy. ACS Biomater. Sci. Eng. 2020, 6, 4096–4105. [Google Scholar]
- Liu, L.; Wang, R.; Wang, C.; Wang, J.; Chen, L.; Cheng, J. Light-triggered release of drug conjugates for an efficient combination of chemotherapy and photodynamic therapy. Biomater. Sci. 2018, 6, 997–1001. [Google Scholar] [PubMed]
- Harnoy, A.J.; Buzhor, M.; Tirosh, E.; Shaharabani, R.; Beck, R.; Amir, R.J. Modular synthetic approach for adjusting the disassembly rates of enzyme-responsive polymeric micelles. Biomacromolecules 2017, 18, 1218–1228. [Google Scholar]
- Phua, S.Z.F.; Xue, C.; Lim, W.Q.; Yang, G.; Chen, H.; Zhang, Y.; Wijaya, C.F.; Luo, Z.; Zhao, Y. Light-responsive prodrug-based supramolecular nanosystems for site-specific combination therapy of cancer. Chem. Mater. 2019, 31, 3349–3358. [Google Scholar]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar]
- Cho, H.J.; Chung, M.; Shim, M.S. Engineered photo-responsive materials for near-infrared-triggered drug delivery. J. Ind. Eng. Chem. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Alemayehu, Y.A.; Fan, W.L.; Bintang Ilhami, F.; Chiu, C.W.; Lee, D.J.; Cheng, C.C. Photosensitive supramolecular micelle-mediated cellular uptake of anticancer drugs enhances the efficiency of chemotherapy. Int. J. Mol. Sci. 2020, 21, 4677. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, H.F.; Barnhart, T.E.; Liu, Z.; Cai, W. Cerenkov radiation induced photodynamic therapy using chlorin e6-loaded hollow mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef] [Green Version]
- Pinto da Silva, L.; Magalhães, C.M.; Núñez-Montenegro, A.; Ferreira, P.J.O.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Study of the combination of self-activating photodynamic therapy and chemotherapy for cancer treatment. Biomolecules 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto da Silva, L.; Núñez-Montenegro, A.; Magalhães, C.M.; Ferreira, P.J.O.; Duarte, D.; González-Berdullas, P.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Single-molecule chemiluminescent photosensitizer for a self-activating and tumor-selective photodynamic therapy of cancer. Eur. J. Med. Chem. 2019, 183, 111683. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Song, C.; Li, Y.; Li, T.; Yang, Y.; Huang, Z.; de la Fuente, J.M.; Ni, J.; Cui, D. Long-circulating drug-dye-based micelles with ultrahigh pH-sensitivity for deep tumor penetration and superior chemo-photothermal therapy. Adv. Funct. Mater. 2020, 30, 1906309. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Krippes, K.; Ritz, S.; Chen, Z.; Best, A.; Butt, H.-J.; Mailänder, V.; Wu, S. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem. Commun. 2015, 51, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Butt, H.J. Near-infrared-sensitive materials based on upconverting nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef]
- Bagheri, A.; Arandiyan, H.; Boyer, C.; Lim, M. Lanthanide-doped upconversion nanoparticles: Emerging intelligent light-activated drug delivery systems. Adv. Sci. 2016, 3, 1500437. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, C.; Chen, G.; Liu, B.; Deng, X.; Wei, Y.; Xia, J.; Xing, B.; Ma, P.; Lin, J. Synthesis and optimization of MoS2@Fe3O4-ICG/Pt(IV) nanoflowers for MR/IR/PA bioimaging and combined PTT/PDT/chemotherapy triggered by 808 nm Laser. Adv. Sci. 2017, 4, 1600540. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.D.; Øverbye, A.; Sahariah, P.; Gaware, V.S.; Høgset, H.; Masson, M.; Høgset, A.; Mælandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-loaded photosensitizer-chitosan nanoparticles for combinatorial chemo- and photodynamic-therapy of cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Morimoto, Y.; Jang, W.-D.; Kataoka, K. Design and development of dendrimer photosensitizer-incorporated polymeric micelles for enhanced photodynamic therapy. Adv. Drug Deliv. Rev. 2009, 61, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Kimura, M.; Nishioka, A.; Kato, H.; Morita, A. Dual wavelength 5-aminolevulinic acid photodynamic therapy using a novel flexible light-emitting diode unit. J. Dermatol. Sci. 2019, 93, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Mohammad-Hadi, L.; MacRobert, A.J.; Loizidou, M.; Yaghini, E. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale 2018, 10, 1570–1581. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Sumer, B.D.; Kessinger, C.W.; Dong, Y.; Huang, G.; Boothman, D.A.; Gao, J. Nanoscopic micelle delivery improves the photophysical properties and efficacy of photodynamic therapy of protoporphyrin IX. J. Control. Release 2011, 151, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Sazgarnia, A.; Rajabi, O.; Soudmand, S.; Esmaily, H.; Sadeghi, H.R. An In vitro study on the photosensitivity of 5-aminolevulinic acid conjugated gold nanoparticles. Photodiagn. Photodyn. Ther. 2013, 10, 382–388. [Google Scholar] [CrossRef]
- Gebeyehu, B.T.; Huang, S.Y.; Lee, A.W.; Chen, J.K.; Lai, J.Y.; Lee, D.J.; Cheng, C.C. Dual stimuli-responsive nucleobase-functionalized polymeric systems as efficient tools for manipulating micellar self-assembly behavior. Macromolecules 2018, 51, 1189–1197. [Google Scholar] [CrossRef]
- Cheng, C.C.; Huang, J.J.; Muhable, A.A.; Liao, Z.S.; Huang, S.Y.; Lee, S.C.; Chiu, C.W.; Lee, D.J. Supramolecular fluorescent nanoparticles functionalized with controllable physical properties and temperature-responsive release behavior. Polym. Chem. 2017, 8, 2292–2298. [Google Scholar] [CrossRef]
- Muhabie, A.A.; Ho, C.H.; Gebeyehu, B.T.; Huang, S.Y.; Chiu, C.W.; Lai, J.-Y.; Lee, D.J.; Cheng, C.C. Dynamic tungsten diselenide nanomaterials: Supramolecular assembly-induced structural transition over exfoliated two-dimensional nanosheets. Chem. Sci. 2018, 9, 5452–5460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.C.; Muhabie, A.A.; Huang, S.Y.; Wu, C.Y.; Gebeyehu, B.T.; Lee, A.-W.; Lai, J.Y.; Lee, D.J. Dual stimuli-responsive supramolecular boron nitride with tunable physical properties for controlled drug delivery. Nanoscale 2019, 11, 10393–10401. [Google Scholar] [CrossRef]
- Bintang Ilhami, F.; Huang, S.Y.; Chen, J.K.; Kao, C.Y.; Cheng, C.C. Multifunctional adenine-functionalized supramolecular micelles for highly selective and effective cancer chemotherapy. Polym. Chem. 2020, 11, 849–856. [Google Scholar] [CrossRef]
- Bintang Ilhami, F.; Alemayehu, Y.A.; Fan, W.L.; Tsai, H.C.; Kao, C.Y.; Cheng, C.C. Adenine-functionalized supramolecular micelles for selective cancer chemotherapy. Macromol. Biosci. 2020, 2000233. [Google Scholar] [CrossRef]
- Muhabie, A.A.; Cheng, C.C.; Huang, J.J.; Liao, Z.S.; Huang, S.-Y.; Chiu, C.-W.; Lee, D.J. Non-covalently functionalized boron nitride mediated by a highly self-assembled supramolecular polymer. Chem. Mater. 2017, 29, 8513–8520. [Google Scholar] [CrossRef]
- Duong, H.; Lee, J.W.; Rhee, J.I. Generation of reactive oxygen species from 5-aminolevulinic acid and Glutamate in cooperation with excited CdSe/ZnS QDs. Proc. SPIE 2014, 9166, 916612. [Google Scholar]
- Feng, Y.; Liu, L.; Hu, S.; Liu, Y.; Ren, Y.; Zhang, X. Förster resonance energy transfer properties of a new type of near-infrared excitation PDT photosensitizer: CuInS2/ZnS quantum dots-5-aminolevulinic acid conjugates. RSC Adv. 2016, 6, 55568–55576. [Google Scholar] [CrossRef]
- Liao, Z.S.; Huang, S.Y.; Huang, J.J.; Chen, J.K.; Lee, A.W.; Lai, J.Y.; Lee, D.J.; Cheng, C.C. Self-assembled pH-responsive polymeric micelles for highly efficient, noncytotoxic delivery of doxorubicin chemotherapy to inhibit macrophage activation: In vitro investigation. Biomacromolecules 2018, 19, 2772–2781. [Google Scholar] [CrossRef]
- Tong, H.; Wang, Y.; Li, H.; Jin, Q.; Ji, J. Dual pH-responsive 5-aminolevulinic acid pseudopolyrotaxane prodrug micelles for enhanced photodynamic therapy. Chem. Commun. 2016, 52, 3966–3969. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ding, L.; Xu, H.-J.; Shen, Z.; Ju, H.; Jia, L.; Bao, L.; Yu, J.-S. Cell-specific and pH-activatable rubyrin-loaded nanoparticles for highly selective near-infrared photodynamic therapy against cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, H.; Jin, Q.; Li, Z.; Li, H.; Ji, J. Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 14596–14605. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, H.S.; Na, K. Photoresponsive micelle-incorporated doxorubicin for chemo-photodynamic therapy to achieve synergistic antitumor effects. Biomacromolecules 2018, 19, 3301–3310. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilhami, F.B.; Peng, K.-C.; Chang, Y.-S.; Alemayehu, Y.A.; Tsai, H.-C.; Lai, J.-Y.; Chiao, Y.-H.; Kao, C.-Y.; Cheng, C.-C. Photo-Responsive Supramolecular Micelles for Controlled Drug Release and Improved Chemotherapy. Int. J. Mol. Sci. 2021, 22, 154. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010154
Ilhami FB, Peng K-C, Chang Y-S, Alemayehu YA, Tsai H-C, Lai J-Y, Chiao Y-H, Kao C-Y, Cheng C-C. Photo-Responsive Supramolecular Micelles for Controlled Drug Release and Improved Chemotherapy. International Journal of Molecular Sciences. 2021; 22(1):154. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010154
Chicago/Turabian StyleIlhami, Fasih Bintang, Kai-Chen Peng, Yi-Shiuan Chang, Yihalem Abebe Alemayehu, Hsieh-Chih Tsai, Juin-Yih Lai, Yu-Hsuan Chiao, Chen-Yu Kao, and Chih-Chia Cheng. 2021. "Photo-Responsive Supramolecular Micelles for Controlled Drug Release and Improved Chemotherapy" International Journal of Molecular Sciences 22, no. 1: 154. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010154