Adsorption of Malachite Green and Alizarin Red S Dyes Using Fe-BTC Metal Organic Framework as Adsorbent
Abstract
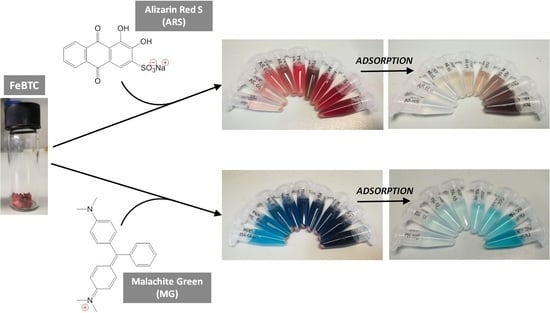
:1. Introduction
2. Results
2.1. Physico-Chemical Characterizations
2.2. Effect of pH on Dyes Adsorption on Fe-BTC MOF
2.3. Adsorption Kinetics
2.4. Adsorption Isotherms
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Synthesis and Characterization of Fe-BTC MOF
4.3. Adsorption Studies
4.3.1. Adsorption Kinetic Models
4.3.2. Adsorption Isotherm Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOF | Metal organic frameworks |
| MG | Malachite green |
| ARS | Alizarin red S |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
References
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M. Sandal reactive dyes decolorization and cytotoxicity reduction using manganese peroxidase immobilized onto polyvinyl alcohol-alginate beads. Chem. Cent. J. 2015, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Ma, B.; Jiang, X.; Ji, Y. Dual function magnetic hydroxyapatite nanopowder for removal of malachite green and Congo red from aqueous solution. Powder Technol. 2016, 302, 207–214. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using tectona grandis sawdust as a very low-cost adsorbent. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Amaterz, E.; Tara, A.; Bouddouch, A.; Taoufyq, A.; Bakiz, B.; Lazar, F.; Gilliot, M.; Benlhachemi, A.; Bazzi, L.; Jbara, O. Hierarchical flower-like SrHPO4 electrodes for the photoelectrochemical degradation of Rhodamine B. J. Appl. Electrochem. 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, D.; Lyu, W.; Xu, H.; Yang, L.; Zhang, Y.; Wang, Z.; Liu, P.; Yan, W.; Tan, G. Design of magnetically assembled electrode (MAE) with Ti/PbO2 and heterogeneous auxiliary electrodes (AEs): The functionality of AEs for efficient electrochemical oxidation. Chem. Eng. J. 2020, 395. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, P.; Wan, C.; Chung, T.S. Polyelectrolyte-promoted Forward Osmosis-Membrane Distillation (FO-MD) hybrid process for dye wastewater treatment. Environ. Sci. Technol. 2012, 46, 6236–6243. [Google Scholar] [CrossRef]
- Yang, C.; Xu, W.; Nan, Y.; Wang, Y.; Hu, Y.; Gao, C.; Chen, X. Fabrication and characterization of a high performance polyimide ultrafiltration membrane for dye removal. J. Colloid Interface Sci. 2020, 562, 589–597. [Google Scholar] [CrossRef]
- Bukman, L.; De Souza, V.R.; Fernandes, N.R.C.; Caetano, W.; Batistela, V.R.; Hioka, N. Reverse micellar extraction of dyes based on fatty acids and recoverable organic solvents. Sep. Purif. Technol. 2020, 242. [Google Scholar] [CrossRef]
- Madhushika, H.G.; Ariyadasa, T.U.; Gunawardena, S.H.P. Biological decolourization of textile industry wastewater by a developed bacterial consortium. Water Sci. Technol. 2020, 80, 1910–1918. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S.; Osma, J.F.; Toca-Herrera, J.L. Removal of synthetic dyes by an eco-friendly strategy. Eng. Life Sci. 2009, 9, 116–123. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, e1703663. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Pitzalis, F.; Carucci, C.; Naseri, M.; Fotouhi, L.; Magner, E.; Salis, A. Lipase Encapsulation onto ZIF-8: A Comparison between Biocatalysts Obtained at Low and High Zinc/2-Methylimidazole Molar Ratio in Aqueous Medium. ChemCatChem 2018, 10, 1578–1585. [Google Scholar] [CrossRef]
- Naseri, M.; Pitzalis, F.; Carucci, C.; Medda, L.; Fotouhi, L.; Magner, E.; Salis, A. Lipase and Laccase Encapsulated on Zeolite Imidazolate Framework: Enzyme Activity and Stability from Voltammetric Measurements. ChemCatChem 2018, 10, 5425–5433. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Samokhvalov, A. Adsorption on Mesoporous Metal-Organic Frameworks in Solution: Aromatic and Heterocyclic Compounds. Chem. Eur. J. 2015, 21, 16726–16742. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z.W. The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Wang, Z.H.; Ma, H.; Li, C.Y.; Zhao, N.; Wang, S. Recent advances on porous organic frameworks for the adsorptive removal of hazardous materials. J. Environ. Sci. (China) 2019, 80, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xu, S.; Liu, J.; He, C.; Xiong, Y.; Feng, P. Highly efficient removal of both cationic and anionic dyes from wastewater with a water-stable and eco-friendly Fe-MOF via host-guest encapsulation. J. Clean. Prod. 2019, 239, 117767. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, M.; De Asua, I.; Ruano, D.; Diaz, K. Direct Synthesis, Structural Features, and Enhanced Catalytic Activity of the Basolite F300-like Semiamorphous Fe-BTC Framework. Cryst. Growth Des. 2015, 15, 4498–4506. [Google Scholar] [CrossRef]
- Gascón, V.; Carucci, C.; Jiménez, M.B.; Blanco, R.M.; Sánchez-Sánchez, M.; Magner, E. Rapid in Situ Immobilization of Enzymes in Metal–Organic Framework Supports under Mild Conditions. ChemCatChem 2017, 9, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Maubert Franco, A.M. Adsorption of azo-dye Orange II from aqueous solutions using a metal-organic framework material: Iron- benzenetricarboxylate. Materials (Basel) 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Wang, Z.; Chen, X.; Jiao, C.; Li, H.; Yu, R. Facile Synthesis of Fe-based MOFs (Fe-BTC) as Efficient Adsorbent for Water Purifications. Chem. Res. Chin. Univ. 2019, 35, 564–569. [Google Scholar] [CrossRef]
- Guesh, K.; Caiuby, C.A.D.; Mayoral, Á.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable Preparation of MIL-100 (Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.H.; Yan, X.P. Metal-organic framework MIL-100 (Fe) for the adsorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Jia, Y.; Jin, Q.; Li, Y.; Sun, Y.; Huo, J.; Zhao, X. Investigation of the adsorption behaviour of different types of dyes on MIL-100 (Fe) and their removal from natural water. Anal. Methods 2015, 7, 1463–1470. [Google Scholar] [CrossRef]
- Tan, F.; Liu, M.; Li, K.; Wang, Y.; Wang, J.; Guo, X.; Zhang, G.; Song, C. Facile synthesis of size-controlled MIL-100 (Fe) with excellent adsorption capacity for methylene blue. Chem. Eng. J. 2015, 281, 360–367. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, C.; Chen, S.; Mei, R.; Wang, X.; Cao, J.; Ma, L.; Zhou, B.; Wei, Q.; Ouyang, G.; et al. Ultrasound enhanced electrochemical oxidation of Alizarin Red S on boron doped diamond(BDD) anode: Effect of degradation process parameters. Chemosphere 2018, 209, 685–695. [Google Scholar] [CrossRef]
- Hanif, S.; Shahzad, A. Removal of chromium(VI) and dye Alizarin Red S (ARS) using polymer-coated iron oxide (Fe3O4) magnetic nanoparticles by co-precipitation method. J. Nanoparticle Res. 2014, 16. [Google Scholar] [CrossRef]
- Legan, L.; Retko, K.; Ropret, P. Vibrational spectroscopic study on degradation of alizarin carmine. Microchem. J. 2016, 127, 36–45. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Culp, S.J.; Beland, F.A. Malachite Green: A Toxicological Review. J. Am. Coll. Toxicol. 1996, 219–238. [Google Scholar] [CrossRef]
- Hashimoto, J.C.; Paschoal, J.A.R.; De Queiroz, J.F.; Reyes, F.G.R. Considerations on the use of malachite green in aquaculture and analytical aspects of determining the residues in fish: A review. J. Aquat. Food Prod. Technol. 2011, 20, 273–294. [Google Scholar] [CrossRef]
- Sacara, A.M.; Nairi, V.; Salis, A.; Turdean, G.L.; Muresan, L.M. Silica-modified Electrodes for Electrochemical Detection of Malachite Green. Electroanalysis 2017, 29, 2602–2609. [Google Scholar] [CrossRef]
- Pangkumhang, B.; Jutaporn, P.; Sorachoti, K.; Khamdahsag, P.; Tanboonchuy, V. Applicability of iron (III) Trimesic (Fe-BTC) to enhance lignin separation from pulp and paper wastewater. Sains Malays. 2019, 48, 199–208. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Li, M.; Si, R. Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Salazar-Aguilar, A.D.; Vega, G.; Casas, J.A.; Vega-Díaz, S.M.; Tristan, F.; Meneses-Rodríguez, D.; Belmonte, M.; Quintanilla, A. Direct hydroxylation of phenol to dihydroxybenzenes by H2O2 and fe-based metal-organic framework catalyst at room temperature. Catalysts 2020, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Majano, G.; Ingold, O.; Yulikov, M.; Jeschke, G.; Pérez-Ramírez, J. Room-temperature synthesis of Fe-BTC from layered iron hydroxides: The influence of precursor organisation. CrystEngComm 2013, 15, 9885–9892. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, Y.; Zhao, F.; Yao, E.; Yi, J.; Xuan, C.; Chen, S. Effects of metal organic framework Fe-BTC on the thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 67308–67314. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Chevreau, H.; Horcajada, P.; Devic, T.; Serre, C.; Garcia, H. Iron(iii) metal-organic frameworks as solid Lewis acids for the isomerization of α-pinene oxide. Catal. Sci. Technol. 2012, 2, 324–330. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Simchi, A.; Shahriyari Far, H. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2020, 81, 405–414. [Google Scholar] [CrossRef]
- Niazi, A.; Ghalie, M.; Yazdanipour, A.; Ghasemi, J. Spectrophotometric determination of acidity constants of Alizarine Red S in water, water-Brij-35 and water-SDS micellar media solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 660–664. [Google Scholar] [CrossRef]
- Chin, Y.P.; Abdul Raof, S.F.; Sinniah, S.; Lee, V.S.; Mohamad, S.; Abdul Manan, N.S. Inclusion complex of Alizarin Red S with β-cyclodextrin: Synthesis, spectral, electrochemical and computational studies. J. Mol. Struct. 2015, 1083, 236–244. [Google Scholar] [CrossRef]
- Cheriaa, J.; Khaireddine, M.; Rouabhia, M.; Bakhrouf, A. Removal of triphenylmethane dyes by bacterial consortium. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghodbane, I.; Kherrrat, R.; Zougar, S.; Lamari, R.; Haddadji, R.; Medjram, M.S. Kinetic study and characterization of a platinum electrode/sensitive membrane for malachite green detection. Sens. Rev. 2018, 38, 335–344. [Google Scholar] [CrossRef]
- Zhang, B.L.; Qiu, W.; Wang, P.P.; Liu, Y.L.; Zou, J.; Wang, L.; Ma, J. Mechanism study about the adsorption of Pb(II) and Cd(II) with iron-trimesic metal-organic frameworks. Chem. Eng. J. 2020, 385, 123507. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal-organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- Embaby, M.S.; Elwany, S.D.; Setyaningsih, W.; Saber, M.R. The adsorptive properties of UiO-66 towards organic dyes: A record adsorption capacity for the anionic dye Alizarin Red S. Chin. J. Chem. Eng. 2018, 26, 731–739. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal-organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The Strengthening Role of the Amino Group in Metal-Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J. Chem. Eng. Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Shi, Z.; Li, L.; Xiao, Y.; Wang, Y.; Sun, K.; Wang, H.; Liu, L. Synthesis of mixed-ligand Cu-MOFs and their adsorption of malachite green. RSC Adv. 2017, 7, 30904–30910. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Ye, J.; Wang, Y.; Qian, X.; Dong, M. Electrospinning Synthesis of ZIF-67/PAN Fibrous Membrane with High-capacity Adsorption for Malachite Green. Fibers Polym. 2019, 20, 2070–2077. [Google Scholar] [CrossRef]
- Li, F.; Zheng, K.; Zhang, H.; Duan, C.; Xi, H. Nanoscale Hierarchically Porous Metal-Organic Frameworks: Facile Synthesis, Mechanism Research, and Application. ACS Sustain. Chem. Eng. 2019, 7, 11080–11087. [Google Scholar] [CrossRef]
- Wu, X.; Huang, M.; Kong, F.; Yu, S. Short communication: Study on the formation of 2-methylimidazole and 4-methylimidazole in the Maillard reaction. J. Dairy Sci. 2015, 98, 8565–8571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlee, C.; Markova, M.; Schrank, J.; Laplagne, F.; Schneider, R.; Lachenmeier, D.W. Determination of 2-methylimidazole, 4-methylimidazole and 2-acetyl-4-(1,2,3,4-tetrahydroxybutyl)imidazole in caramel colours and cola using LC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 927, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Gascón, V.; Jiménez, M.B.; Blanco, R.M.; Sanchez-Sanchez, M. Semi-crystalline Fe-BTC MOF material as an efficient support for enzyme immobilization. Catal. Today 2018, 304, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. Computations from nitrogen isotherm. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Jamal, M.M.E.; Mousaoui, A.M.; Naoufal, D.M. Effect of Operating Parameters on Electrochemical Degradation of Alizarin Red S on Pt and BDD Electrodes. Port. Electrochim. Acta 2014, 32, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Yuan, X.; Yang, J.; Yuan, J.; Shi, J.; Wang, Y.; Chen, Y. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy A chemometric-assisted method for the simultaneous determination of malachite green and crystal violet in water based on absorbance–pH data generated by a homemade pH gradient apparatu. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 403–408. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Lv, L.; Pan, B.C.; Zhang, Q.J.; Zhang, W.M.; Zhang, Q.X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Delpiano, G.R.; Zanda, D.; Piludu, M.; Sanjust, E.; Monduzzi, M.; Salis, A. Adsorption of Cu2+ and Zn2+ on SBA-15 mesoporous silica functionalized with triethylenetetramine chelating agent. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses. Chemosphere 2005, 61, 492–501. [Google Scholar] [CrossRef]






| qe exp (mg g−1) | Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k′ (min−1) | qe cal (mg g−1) | R | k″ (g mg−1 min−1) | qe cal (mg g−1) | R | ki (g mg−1 min−1/2) | xi (mg g−1) | R | ||
| ARS | 80.39 | 5.98 × 10−3 | 12.78 | 0.946 | 4.29 × 10−3 | 81.09 | 0.992 | 27.77 1.10 0.33 | 10.44 64.81 72.38 | 0.885 0.873 0.999 |
| MG | 177.28 | 1.3210−2 | 1.75 | 0.707 | 3.98 × 10−2 | 177.31 | 1 | 11.33 0.58 0.02 | 149.38 172.51 176.88 | 1 0.828 0.434 |
| Temkin | Freundlich | Langmuir | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| bT | AT (L mg−1) | R | KF (L mg−1) | 1/n | R | KL (L mg−1) | qe,max (mg g−1) | R | ∆G° (KJ mol−1) | |
| ARS | 157.59 | 1.28·105 | 0.982 | 3.85·103 | 0.529 | 0.910 | 9.30·103 | 79.88 | 0.995 | −54.21 |
| MG | 79.34 | 9.87·105 | 0.967 | 63.77·103 | 0.624 | 0.909 | 51.56·103 | 187.24 | 0.967 | −58.61 |
| Synthesis | Kinetic | Isotherm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adsorbent | T (°C) | t (h) | Solvent | Dye | qe(exp) (mg/g) | t (min) | k″ (g∙mg min) | Model | K (L∙mg−1) | Ref. |
| Fe-BTC | 25 | <1 | H2O | Alizarin red S | 80 | 30 | 4.29 × 10−3 | Langmuir | 9.30∙103 | This work |
| Malachite green | 177 | 30 | 3.98 × 10−2 | Langmuir | 51.56∙103 | |||||
| Fe-BTC | 150 | 12 | H2O | Malachite green | 205 | 120 | 6.67∙10−3 | Freundlich | 6.49 | [32] |
| UiO-66 | 120 | 1 | DMF | Alizarin red S | 400 | 36 | 2.3∙10−4 | Langmuir | 0.06 | [55] |
| Cu-BTC | 100 | 10 | EtOH/DMF | Methylene blue | 4.68 | 10 | 42.39 | Langmuir | 1.89 | [57] |
| Mil-53(Al) -NH2 | 150 | 24 | DMF/H20 | Malachite green | 37.8 | 200 | - | Langmuir | 0.29 | [59] |
| Methylene blue | 45.2 | 200 | - | Langmuir | 0.67 | |||||
| Cu-BTC/BDC | 120 | 12 | EtOH | Malachite green | 185 | - | - | Freundlich | - | [60] |
| ZIF-67/PAN | 25 | <1 | H2O | Malachite green | 2545 | 60 | 2.7∙10−3 | Langmuir | 0.05 | [61] |
| NH-ZIF-67 | 25 | <1 | MeOH | Malachite green | 114.1 | 240 | - | - | - | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delpiano, G.R.; Tocco, D.; Medda, L.; Magner, E.; Salis, A. Adsorption of Malachite Green and Alizarin Red S Dyes Using Fe-BTC Metal Organic Framework as Adsorbent. Int. J. Mol. Sci. 2021, 22, 788. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020788
Delpiano GR, Tocco D, Medda L, Magner E, Salis A. Adsorption of Malachite Green and Alizarin Red S Dyes Using Fe-BTC Metal Organic Framework as Adsorbent. International Journal of Molecular Sciences. 2021; 22(2):788. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020788
Chicago/Turabian StyleDelpiano, Giulia Rossella, Davide Tocco, Luca Medda, Edmond Magner, and Andrea Salis. 2021. "Adsorption of Malachite Green and Alizarin Red S Dyes Using Fe-BTC Metal Organic Framework as Adsorbent" International Journal of Molecular Sciences 22, no. 2: 788. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020788