Potentiometric Study of Carbon Nanotube/Surfactant Interactions by Ion-Selective Electrodes. Driving Forces in the Adsorption and Dispersion Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carbon Nanotube/Surfactant Interactions: Adsorption Process
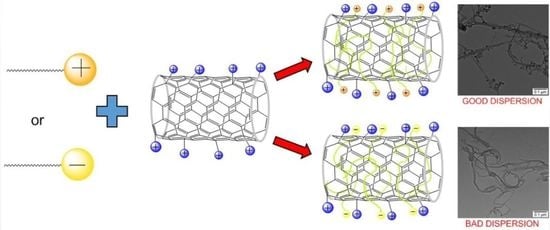
2.2. Carbon Nanotube/Surfactant Interactions: Dispersion Process
3. Material and Methods
3.1. Materials
3.2. Dispersion of Carbon Nanotubes
3.3. Potentiometric Measurements
3.4. Transmission Electron Microscopy (TEM)
3.5. Zeta-Potential Measurements
3.6. Dynamic Light-Scattering Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oberlin, A.; Endo, M.; Koyam, T. Filamentous growth of carbon through benzene decomposition. J. Crys. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Jia, X.; Wei, F. Advances in production and applications of carbon nanotubes. In Single-Walled Carbon Nanotubes; Li, Y., Maruyama, S., Eds.; Topics in Current Chemistry Collections; Springer: Cham, Switzerland, 2019; pp. 299–333. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental remediation applications of carbon nanotubes and graphene oxide: Adsorption and catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Hassana, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and chances. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Feng, L.; Dong, S.; Hao, J.; Yu, Q. Carbon nanotubes modified by a paramagnetic cationic surfactant for migration of DNA and proteins. Colloids Surf. A 2018, 559, 201–208. [Google Scholar] [CrossRef]
- Rodríguez, C.; Briano, S.; Leiva, E. Increased adsorption of heavy metal ions in multi-walled carbon nanotubes with improved dispersion stability. Molecules 2020, 25, 3106. [Google Scholar] [CrossRef]
- Tham, G.X.; Fisher, A.C.; Webster, R.D. Voltammetric studies on surfacemodified electrodes with functionalised carbon nanotubes under different dispersion conditions. Electrochim. Acta 2020, 357, 1–15. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Wong, E.H. Elucidation of antimicrobial activity of non-covalently dispersed carbon nanotubes. Materials 2020, 13, 1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Yang, K.; Zhang, S.; Chefetz, B.; Zhao, J.; Mashayekhi, H.; Xing, B. Dispersant selection for nanomaterials: Insight into dispersing functionalized carbon nanotubes by small polar aromatic organic molecules. Carbon 2015, 91, 494–505. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Zhang, L.; Wang, G.; Chen, G.; Lin, D.; Xing, B. Dispersion and stability of multi-walled carbon nanotubes in water as affected by humic acids. J. Mol. Liq. 2019, 279, 361–369. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Hodak, M.; Lee, R.S. Carbon nanotubes, buckyballs, ropes, and a universal graphitic potential. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 62, 13104–13110. [Google Scholar] [CrossRef]
- Lu, K.L.; Lago, R.M.; Chen, Y.K.; Green, M.L.H.; Harris, P.J.F.; Tsang, S.C. Mechanical Damage of Carbon Nanotubes by Ultrasound. Carbon 1996, 34, 814–816. [Google Scholar] [CrossRef]
- Gerstel, P.; Klumpp, S.; Hennrich, F.; Poschlad, A.; Meded, V.; Blasco, E.; Wenzel, W.; Kappes, M.M.; Barner-Kowollik, C. Highly Selective Dispersion of single-walled carbon nanotubes via polymer wrapping: A combinatorial study via modular conjugation. ACS Macro Lett. 2014, 3, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.V.; Cividanes, L.D.S.; Brito, F.S.; de Menezes, B.R.C.; Franceschi, W.; Simonetti, E.A.N.; Thim, G.P. Functionalizing Graphene and Carbon Nanotubes. A Review; SpringerBriefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Naqvi, S.T.R.; Rasheed, T.; Hussain, D.; Najam ul Haq, M.; Majeed, S.; Shafi, S.; Ahmed, N.; Nawaz, R. Modification strategies for improving the solubility/dispersion of carbon nanotubes. J. Mol. Liq. 2020, 297, 1–12. [Google Scholar] [CrossRef]
- Bricha, M.; El Mabrouk, K. Effect of surfactants on the degree of dispersion of MWNTs in ethanol solvent. Colloids Surf. A 2019, 561, 57–69. [Google Scholar] [CrossRef]
- Rice, N.A.; Subrahmanyam, A.V.; Coleman, B.R.; Adronov, A. Effect of induction on the dispersion of semiconducting and metallic single-walled carbon nanotubes using conjugated polymers. Macromolecules 2015, 48, 5155–5161. [Google Scholar] [CrossRef]
- Yang, Y.; Sharma, A.; Noetinger, G.; Zheng, M.; Jagota, A. Pathway-dependent structures of DNA-wrapped carbon nanotubes: Direct sonication vs surfactant/DNA exchange. J. Phys. Chem. C 2020, 124, 9045–9055. [Google Scholar] [CrossRef]
- Sohrabi, B.; Poorgholami-Bejarpasi, N.; Nayeri, N. Dispersion of carbon nanotubes using mixed surfactants: Experimental and molecular dynamics simulation studies. J. Phys. Chem. B 2014, 118, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. Surfactant-aided dispersion of carbon nanomaterials in aqueous solution. Phys. Fluids 2019, 31, 1–24. [Google Scholar] [CrossRef]
- Abreu, B.; Rocha, J.; Fernandes, R.M.F.; Regev, O.; Furó, I.; Marques, E.F. Gemini surfactants as efficient dispersants of multiwalled carbon nanotubes: Interplay of molecular parameters on nanotube dispersibility and debundling. J. Colloid Interface Sci. 2019, 547, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, J.; Gan, X.; Wang, H.; Su, X.; Xi, C. A systematic investigation of dispersion concentration and particle size distribution of multi-wall carbon nanotubes in aqueous solutions of various dispersants. Colloids Surf. A 2020, 589, 1–11. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Mackiewicz, N.; Surendran, G.; Remita, H.; Keita, B.; Zhang, G.; Nadjo, L.; Hagège, A.; Doris, E.; Mioskowski, C. Supramolecular self-assembly of amphiphiles on carbon nanotubes: A versatile strategy for the construction of CNT/metal nanohybrids, application to electrocatalysis. J. Am. Chem. Soc. 2008, 130, 8110–8111. [Google Scholar] [CrossRef]
- Yurekli, K.; Mitchell, C.A.; Krishnamoorti, R. Small-angle neutron scattering from surfactant-assisted aqueous dispersions of carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 9902–9903. [Google Scholar] [CrossRef]
- Tummala, N.R.; Striolo, A. SDS surfactants on carbon nanotubes: Aggregate morphology. ACS Nano 2009, 3, 595–602. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Germani, R.; Del Canto, E.; Giordani, S.; Savelli, G.; Fontana, A. Effect of surfactant structure on carbon nanotube sidewall adsorption. Eur. J. Org. Chem. 2011, 5641–5648. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Camber, S.; Germani, R.; Di Profio, P.; Fontana, A. Dispersion of SWCNTs with imidazolium-rich surfactants. Langmuir 2014, 30, 3979–3987. [Google Scholar] [CrossRef] [PubMed]
- Hongzhi Cui, H.; Yan, X.; Monasterio, M.; Xing, F. Effects of various surfactants on the dispersion of MWCNTs–OH in aqueous solution. Nanomaterials 2017, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Madni, I.; Hwang, C.-H.; Park, S.-D.; Choa, Y.-H.; Kim, H.-T. Mixed surfactant system for stable suspension of multiwalled carbon nanotubes. Colloids Surf. A 2010, 358, 101–107. [Google Scholar] [CrossRef]
- López-López, M.; Bernal, E.; Moyá, M.L.; Sanchez, F.; López-Cornejo, P. Study of ionic surfactants interactions with carboxylated single-walled carbon nanotubes by using ion-selective electrodes. Electrochem. Commun. 2016, 67, 31–34. [Google Scholar] [CrossRef]
- Angelikopoulosa, P.; Bock, H. The science of dispersing carbon nanotubes with surfactants. Phys. Chem. Chem. Phys. 2012, 14, 9546–9557. [Google Scholar] [CrossRef]
- Wang, H. Dispersing carbon nanotubes using surfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 364–371. [Google Scholar] [CrossRef]
- López-López, M.; López-Cornejo, P.; Martín, V.I.; Ostos, F.J.; Checa-Rodríguez, C.; Prados-Carvajal, R.; Lebrón, J.A.; Huertas, P.; Moyá, M.L. Importance of hydrophobic interactions in the single-chained cationic surfactant-DNA complexation. J. Colloid Interface Sci. 2018, 521, 197–205. [Google Scholar] [CrossRef]
- Lin, C.E. Determination of critical micelle concentration of surfactants by capillary electrophoresis. J. Chromatogr. A 2004, 1037, 467–478. [Google Scholar] [CrossRef]
- Cifuentes, A.; Bernal, J.L.; Diez-Masa, J.C. Determination of critical micelle concentration values using capillary electrophoresis instrumentation. Anal. Chem. 1997, 69, 4271–4274. [Google Scholar] [CrossRef]








| CNT/Surfactant | ζ/mv * | dH/nm ** | PDI ** |
|---|---|---|---|
| SWCNT/DoTAB | 4.1 ± 0.2 | 1180 ± 30 | 0.455 |
| SWCNT/SDS | −3.9 ± 0.3 | 1031 ± 25 | 0.556 |
| MWCNT/DoTAB | 1.7 ± 0.1 | 1483 ± 27 | 0.598 |
| MWCNT/SDS | −4.5 ± 0.3 | 1194 ± 32 | 0.664 |
| SWCNT-NH2 | 13.3 ± 0.4 | 928 ± 15 | 0.470 |
| SWCNT-NH2/DoTAB | 22.5 ± 0.2 | 920 ± 12 | 0.391 |
| SWCNT-NH2/SDS | 8.6 ± 0.3 | 958 ± 16 | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostos, F.J.; Lebrón, J.A.; Moyá, M.L.; Bernal, E.; Flores, A.; Lépori, C.; Maestre, Á.; Sánchez, F.; López-Cornejo, P.; López-López, M. Potentiometric Study of Carbon Nanotube/Surfactant Interactions by Ion-Selective Electrodes. Driving Forces in the Adsorption and Dispersion Processes. Int. J. Mol. Sci. 2021, 22, 826. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020826
Ostos FJ, Lebrón JA, Moyá ML, Bernal E, Flores A, Lépori C, Maestre Á, Sánchez F, López-Cornejo P, López-López M. Potentiometric Study of Carbon Nanotube/Surfactant Interactions by Ion-Selective Electrodes. Driving Forces in the Adsorption and Dispersion Processes. International Journal of Molecular Sciences. 2021; 22(2):826. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020826
Chicago/Turabian StyleOstos, Francisco José, José Antonio Lebrón, María Luisa Moyá, Eva Bernal, Ana Flores, Cristian Lépori, Ángeles Maestre, Francisco Sánchez, Pilar López-Cornejo, and Manuel López-López. 2021. "Potentiometric Study of Carbon Nanotube/Surfactant Interactions by Ion-Selective Electrodes. Driving Forces in the Adsorption and Dispersion Processes" International Journal of Molecular Sciences 22, no. 2: 826. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020826