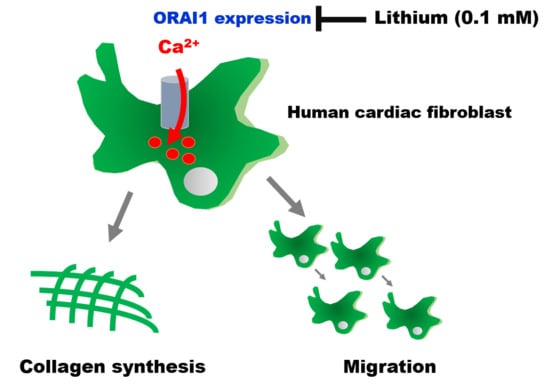
Lithium Reduces Migration and Collagen Synthesis Activity in Human Cardiac Fibroblasts by Inhibiting Store-Operated Ca2+ Entry
Abstract
:1. Introduction
2. Results
2.1. Effects of Therapeutic Trough Levels of Lithium on Cardiac Fibroblasts
2.2. Therapeutic Trough Levels of Lithium Reduce Store-Operated Ca2+ Entry
2.3. Therapeutic Trough Levels of Lithium Decrease Orai1 Expression
2.4. Orai1 Inhibitor 2-APB on the Effects of Lithium in Cardiac Fibroblasts
2.5. Therapeutic Trough Levels of Lithium Increase Phosphorylated Akt Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Migration Assay
4.3. Cell Proliferation Assay
4.4. Western Blotting
4.5. Soluble Collagen Measurement
4.6. Transfection of siRNA into Human Cardiac Fibroblast
4.7. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR) Analysis
4.8. Calcium Fluorescence Imaging
4.9. Immunofluorescence Microscopic Imaging
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, K.; Winkleby, M.A.; Sundquist, J. Comorbidities and mortality in bipolar disorder: A Swedish national cohort study. JAMA Psychiatry 2013, 70, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Teixeira, A.L.; Cao, F.; Gildengers, A.; Soares, J.C.; Butters, M.A.; Reynolds, C.F., 3rd. History of bipolar disorder and the risk of dementia: A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 2017, 25, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westman, J.; Hallgren, J.; Wahlbeck, K.; Erlinge, D.; Alfredsson, L.; Osby, U. Cardiovascular mortality in bipolar disorder: A population-based cohort study in Sweden. BMJ Open 2013, 3, e002373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.H.; Tsai, S.Y.; Pan, C.H.; Chang, H.M.; Chen, Y.L.; Su, S.S.; Chen, C.C.; Kuo, C.J. Incidence and risk factors of sudden cardiac death in bipolar disorder across the lifespan. J. Affect. Disord. 2020, 274, 210–217. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Vilahur, G.; Juan-Babot, O.; Peña, E.; Oñate, B.; Casaní, L.; Badimon, L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011, 50, 522–533. [Google Scholar] [CrossRef]
- Feng, J.; Armillei, M.K.; Yu, A.S.; Liang, B.T.; Runnels, L.W.; Yue, L. Ca2+ signaling in cardiac fibroblasts and fibrosis-associated heart diseases. J. Cardiovasc. Dev. Dis. 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Lee, T.I.; Chen, Y.J. Vascular endothelial growth factor enhances profibrotic activities through modulation of calcium homeostasis in human atrial fibroblasts. Lab. Investig. 2020, 100, 285–296. [Google Scholar] [CrossRef]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Yeh, Y.H.; Chen, Y.J. Factor Xa inhibition by rivaroxaban regulates fibrogenesis in human atrial fibroblasts with modulation of nitric oxide synthesis and calcium homeostasis. J. Mol. Cell. Cardiol. 2018, 123, 128–138. [Google Scholar] [CrossRef]
- Bartoli, F.; Bailey, M.A.; Rode, B.; Mateo, P.; Antigny, F.; Bedouet, K.; Gerbaud, P.; Gosain, R.; Plante, J.; Norman, K.; et al. Orai1 channel inhibition preserves left ventricular systolic function and normal Ca2+ handling after pressure overload. Circulation 2020, 141, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Eder, P. Cardiac remodeling and disease: SOCE and TRPC signaling in cardiac pathology. Adv. Exp. Med. Biol. 2017, 993, 505–521. [Google Scholar] [PubMed]
- Ross, G.R.; Bajwa, T., Jr.; Edwards, S.; Emelyanova, L.; Rizvi, F.; Holmuhamedov, E.L.; Werner, P.; Downey, F.X.; Tajik, A.J.; Jahangir, A. Enhanced store-operated Ca2+ influx and ORAI1 expression in ventricular fibroblasts from human failing heart. Biol. Open 2017, 6, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Mohis, M.; Edwards, S.; Ryan, S.; Rizvi, F.; Tajik, A.J.; Jahangir, A.; Ross, G.R. Aging-related increase in store-operated Ca 2+ influx in human ventricular fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H83–H91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhi, G.S.; Gessler, D.; Outhred, T. The use of lithium for the treatment of bipolar disorder: Recommendations from clinical practice guidelines. J. Affect. Disord. 2017, 217, 266–280. [Google Scholar] [CrossRef]
- Kessing, L.V.; Gerds, T.A.; Knudsen, N.N.; Jørgensen, L.F.; Kristiansen, S.M.; Voutchkova, D.; Ernstsen, V.; Schullehner, J.; Hansen, B.; Andersen, P.K.; et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry 2017, 74, 1005–1010. [Google Scholar] [CrossRef]
- Zarse, K.; Terao, T.; Tian, J.; Iwata, N.; Ishii, N.; Ristow, M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr. 2011, 50, 387–389. [Google Scholar] [CrossRef] [Green Version]
- Voors, A.W. Lithium in the drinking water and atherosclerotic heart death; epidemiologic argument for protective effect. Am. J. Epidemiol. 1970, 92, 164–171. [Google Scholar] [CrossRef]
- Prosser, J.M.; Fieve, R.R. Patients receiving lithium therapy have a reduced prevalence of neurological and cardiovascular disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 39–44. [Google Scholar] [CrossRef]
- Lan, C.C.; Liu, C.C.; Lin, C.H.; Lan, T.Y.; McInnis, M.G.; Chan, C.H.; Lan, T.H. A reduced risk of stroke with lithium exposure in bipolar disorder: A population-based retrospective cohort study. Bipolar Disord. 2015, 17, 705–714. [Google Scholar] [CrossRef]
- Chen, P.H.; Chao, T.F.; Kao, Y.H.; Chen, Y.J. Lithium interacts with cardiac remodeling: The fundamental value in the pharmacotherapy of bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Pelzl, L.; Elsir, B.; Sahu, I.; Bissinger, R.; Singh, Y.; Sukkar, B.; Honisch, S.; Schoels, L.; Jemaà, M.; Lang, E.; et al. Lithium sensitivity of store operated Ca2+ entry and survival of fibroblasts isolated from chorea-acanthocytosis patients. Cell. Physiol. Biochem. 2017, 42, 2066–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelzl, L.; Hauser, S.; Elsir, B.; Sukkar, B.; Sahu, I.; Singh, Y.; Höflinger, P.; Bissinger, R.; Jemaà, M.; Stournaras, C.; et al. Lithium sensitive ORAI1 expression, store operated Ca2+ entry and suicidal death of neurons in chorea-acanthocytosis. Sci. Rep. 2017, 7, 6457. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, M.J.; Corson, T.W.; Sibony, D.; Cooke, R.G.; Parikh, S.V.; Pennefather, P.S.; Li, P.P.; Warsh, J.J. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology 2004, 29, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, E.M.; Aubry, J.M. Lithium: Updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring. CNS Drugs 2009, 23, 331–349. [Google Scholar] [CrossRef]
- Findling, R.L.; Landersdorfer, C.B.; Kafantaris, V.; Pavuluri, M.; McNamara, N.K.; McClellan, J.; Frazier, J.A.; Sikich, L.; Kowatch, R.; Lingler, J.; et al. First-dose pharmacokinetics of lithium carbonate in children and adolescents. J. Clin. Psychopharmacol. 2010, 30, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Pandit, V.; Nesbitt, S.R.; Kim, D.Y.; Mixon, A.; Kotha, S.P. Combinatorial therapy using negative pressure and varying lithium dosage for accelerated wound healing. J. Mech. Behav. Biomed. Mater. 2015, 44, 173–178. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Hao, J.; Li, N.; Tang, J.; Hao, L. Up-regulation of microRNA-93 inhibits TGF-β1-induced EMT and renal fibrogenesis by down-regulation of Orai1. J. Pharmacol. Sci. 2018, 136, 218–227. [Google Scholar] [CrossRef]
- Mai, X.; Shang, J.; Liang, S.; Yu, B.; Yuan, J.; Lin, Y.; Luo, R.; Zhang, F.; Liu, Y.; Lv, X.; et al. Blockade of Orai1 store-operated calcium entry protects against renal fibrosis. J. Am. Soc. Nephrol. 2016, 27, 3063–3078. [Google Scholar] [CrossRef] [Green Version]
- Falcón, D.; Galeano-Otero, I.; Calderón-Sánchez, E.; Del Toro, R.; Martín-Bórnez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP Channels: Current perspectives in the adverse cardiac remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef]
- Inoue, R.; Kurahara, L.H.; Hiraishi, K. TRP channels in cardiac and intestinal fibrosis. Semin. Cell Dev. Biol. 2019, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Andreopoulos, S.; Wasserman, M.; Woo, K.; Li, P.P.; Warsh, J.J. Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharmacogenom. J. 2004, 4, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uemura, T.; Green, M.; Warsh, J.J. Chronic LiCl pretreatment suppresses thrombin-stimulated intracellular calcium mobilization through TRPC3 in astroglioma cells. Bipolar Disord. 2016, 18, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Lin, S.Z.; Chang, N.C. Effect of lithium on ventricular remodelling in infarcted rats via the Akt/mTOR signalling pathways. Biosci. Rep. 2017, 37, BSR20160257. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.C.; Boada, F.; Keshavan, M.S. Brain lithium measurements with (7)Li magnetic resonance spectroscopy (MRS): A literature review. Eur. Neuropsychopharmacol. 2000, 10, 151–158. [Google Scholar] [CrossRef]
- Ma, Z.G.; Yuan, Y.P.; Wu, H.M.; Zhang, X.; Tang, Q.Z. Cardiac fibrosis: New insights into the pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Frye, M.A.; Shelton, R.C. Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacology 2012, 37, 77–101. [Google Scholar] [CrossRef]
- Laeremans, H.; Rensen, S.S.; Ottenheijm, H.C.; Smits, J.F.; Blankesteijn, W.M. Wnt/frizzled signalling modulates the migration and differentiation of immortalized cardiac fibroblasts. Cardiovasc. Res. 2010, 87, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhu, L.J.; Waaga-Gasser, A.M.; Ding, Y.; Cao, M.; Jadhav, S.J.; Kirollos, S.; Shekar, P.S.; Padera, R.F.; Chang, Y.C.; et al. The axis of local cardiac endogenous Klotho-TGF-β1-Wnt signaling mediates cardiac fibrosis in human. J. Mol. Cell. Cardiol. 2019, 136, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Kawano, H.; Do, Y.S.; Kawano, Y.; Starnes, V.; Barr, M.; Law, R.E.; Hsueh, W.A. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 2000, 101, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Jiang, J.; Yue, Z.; Liu, S.; Ma, Y.; Yu, N.; Gao, Y.; Sun, S.; Chen, S.; Liu, P. Store-Operated Ca 2+ Entry (SOCE) contributes to angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J. Pharmacol. Sci. 2016, 132, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simo-Cheyou, E.R.; Tan, J.J.; Grygorczyk, R.; Srivastava, A.K. STIM-1 and ORAI-1 channel mediate angiotensin-II-induced expression of Egr-1 in vascular smooth muscle cells. J. Cell. Physiol. 2017, 232, 3496–3509. [Google Scholar] [CrossRef] [PubMed]
- Kupfahl, C.; Pink, D.; Friedrich, K.; Zurbrügg, H.R.; Neuss, M.; Warnecke, C.; Fielitz, J.; Graf, K.; Fleck, E.; Regitz-Zagrosek, V. Angiotensin II directly increases transforming growth factor b1 and osteopontin and indirectly affects collagen mRNA expression in the human heart. Cardiovasc. Res. 2000, 46, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.; Pantev, E.; Möller, S.; Erlinge, D.; Edvinsson, L. Angiotensin II type 1 receptors stimulate protein synthesis in human cardiac fibroblasts via a Ca2+-sensitive PKC-dependent tyrosine kinase pathway. Acta Physiol. Scand. 2000, 168, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Bouzegrhane, F.; Thibault, G. Is angiotensin II a proliferative factor of cardiac fibroblasts? Cardiovasc. Res. 2002, 53, 304–312. [Google Scholar] [CrossRef]
- Bellomo, R.; Wunderink, R.G.; Szerlip, H.; English, S.W.; Busse, L.W.; Deane, A.M.; Khanna, A.K.; McCurdy, M.T.; Ostermann, M.; Young, P.J.; et al. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit. Care 2020, 24, 43. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Lareu, R.R.; Arsianti, I.; Subramhanya, H.K.; Yanxian, P.; Raghunath, M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: A preliminary study. Tissue Eng. 2007, 13, 385–391. [Google Scholar] [CrossRef]
- Chen, C.Z.; Raghunath, M. Focus on collagen: In vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair 2009, 2, 7. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Chung, C.-C.; Lin, Y.-F.; Kao, Y.-H.; Chen, Y.-J. Lithium Reduces Migration and Collagen Synthesis Activity in Human Cardiac Fibroblasts by Inhibiting Store-Operated Ca2+ Entry. Int. J. Mol. Sci. 2021, 22, 842. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020842
Chen P-H, Chung C-C, Lin Y-F, Kao Y-H, Chen Y-J. Lithium Reduces Migration and Collagen Synthesis Activity in Human Cardiac Fibroblasts by Inhibiting Store-Operated Ca2+ Entry. International Journal of Molecular Sciences. 2021; 22(2):842. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020842
Chicago/Turabian StyleChen, Pao-Huan, Cheng-Chih Chung, Yuan-Feng Lin, Yu-Hsun Kao, and Yi-Jen Chen. 2021. "Lithium Reduces Migration and Collagen Synthesis Activity in Human Cardiac Fibroblasts by Inhibiting Store-Operated Ca2+ Entry" International Journal of Molecular Sciences 22, no. 2: 842. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020842