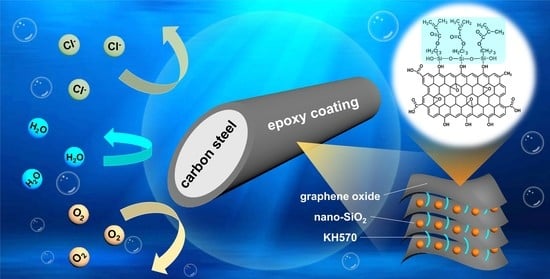
Nano-SiO2 and Silane Coupling Agent Co-Decorated Graphene Oxides with Enhanced Anti-Corrosion Performance of Epoxy Composite Coatings
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Morphology Characterization of Decorated GO
2.2. Structural Characterization of Decorated GO
2.3. Modification Effect Analysis of Decorated GO
2.4. Morphology Characterization of Decorated GO-Filled Epoxy Coating
2.5. Anti-Corrosion Performance of Decorated GO-Filled Epoxy Coating
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO
3.3. Preparation of G-S
3.4. Preparation of G-K and G-S-K
3.5. Materials Characterization
3.6. Oil–Water Mixing Experiment
3.7. Preparation of Epoxy Coating
3.8. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Yi, D.; Wang, Z.; Yu, J.; Zhang, Z.; Qiao, R.; Sun, Z.; Hu, Z.; Gao, P.; Peng, H.; et al. Greatly enhanced anticorrosion of Cu by commensurate graphene coating. Adv. Mater. 2018, 30, 1702944. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Shen, T.; Chen, H.; Lu, J.; Yang, H.-C.; Li, W. Slippery liquid-infused porous surfaces (SLIPSs): A perfect solution to both marine fouling and corrosion? J. Mater. Chem. A 2020, 8, 7536–7547. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.Q.; Zhao, J.B.; Li, X. Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives. Corros. Sci. 2020, 165, 108416. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Taheri, N.N.; Ramezanzadeh, B.; Mahdavian, M. Application of layer-by-layer assembled graphene oxide nanosheets/polyaniline/zinc cations for construction of an effective epoxy coating anti-corrosion system. J. Alloys Compd. 2019, 800, 532–549. [Google Scholar] [CrossRef]
- Zheng, S.; Bellido-Aguilar, D.A.; Wu, X.; Zhan, X.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Durable waterborne hydrophobic bio-epoxy coating with improved anti-icing and self-cleaning performance. ACS Sustain. Chem. Eng. 2019, 7, 641–649. [Google Scholar] [CrossRef]
- Yan, H.; Cai, M.; Li, W.; Fan, X.; Zhu, M. Amino-functionalized Ti3C2Tx with anti-corrosive/wear function for waterborne epoxy coating. J. Mater. Sci. Technol. 2020, 54, 144–159. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Zhang, S.; Zhang, F.; Liu, C.; Zhang, N.; Yao, H.; Shi, Y. Using 3-isocyanatopropy ltrimethoxysilane to decorate graphene oxide with nano-titanium dioxide for enhancing the anti-corrosion properties of epoxy coating. Polymers 2020, 12, 837. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, C.; Zhang, X.; Lin, D.; Xu, F.; Zhu, Y.; Wang, H.; Gong, J.; Wang, T. Highly orientated graphene/epoxy coating with exceptional anti-corrosion performance for harsh oxygen environments. Corros. Sci. 2020, 176, 109049. [Google Scholar] [CrossRef]
- Sari, M.G.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an epoxy composite coating with enhanced corrosion resistance through impregnation of functionalized graphene oxide-co-montmorillonite Nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Ziat, Y.; Hammi, M.; Zarhri, Z.; Laghlimi, C. Epoxy coating modified with graphene: A promising composite against corrosion behavior of copper surface in marine media. J. Alloys Compd. 2020, 820, 153380. [Google Scholar] [CrossRef]
- Zhang, X.; Si, Y.; Mo, J.; Guo, Z. Robust micro-nanoscale flowerlike ZnO/epoxy resin superhydrophobic coating with rapid healing ability. Chem. Eng. J. 2017, 313, 1152–1159. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, M.; Li, W.; Xu, G.; Huang, X.; Yi, X.; Chen, J.; Wu, Y. Study on preparation and properties of CeO2/epoxy resin composite coating on sintered NdFeB magnet. J. Rare Earth 2018, 36, 544–551. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Zhang, Y.; Meng, G.; Zhang, T.; Wang, F. Using high-temperature mechanochemistry treatment to modify iron oxide and improve the corrosion performance of epoxy coating-II. Effect of grinding temperature. Corros. Sci. 2015, 90, 451–462. [Google Scholar] [CrossRef]
- Hu, Y.; Du, G.P.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity. Compos. Sci. Technol. 2016, 124, 36–43. [Google Scholar] [CrossRef]
- Jlassi, K.; Radwan, A.B.; Sadasivuni, K.K.; Mrlik, M.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Anti-corrosive and oil sensitive coatings based on epoxy/polyaniline/magnetite-clay composites through diazonium interfacial chemistry. Sci. Rep. 2018, 8, 13369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Wang, W.; Zhang, L.; Vinokurov, V.; Stavitskaya, A.; Lvov, Y. Development of marine antifouling epoxy coating enhanced with clay nanotubes. Materials 2019, 12, 4195. [Google Scholar] [CrossRef] [Green Version]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Manipulating graphene oxide nanocontainer with benzimidazole and cerium ions: Application in epoxy-based nanocomposite for active corrosion protection. Corros. Sci. 2020, 165, 108379. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, X.; Jiang, Q.; Sun, Z.; Wang, P.; Qiu, Y.; Liu, W. Influence of graphene oxide with different oxidation levels on the properties of epoxy composites. Compos. Sci. Technol. 2018, 161, 74–84. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, Q.; Cheng, L.; Wu, H.; Zhao, H.; Wang, L. Self-alignment of cationic graphene oxide nanosheets for anticorrosive reinforcement of epoxy coatings. Chem. Eng. J. 2020, 389, 124435. [Google Scholar] [CrossRef]
- Guo, T.; Li, H.; Ma, X.; Shi, L.; Wang, W.; Zhang, W.; Li, L. Hyperbranched polyester modified graphene oxide on anti-corrosion performance of epoxy composite coatings for electric power system. Plast. Rubber Compos. 2020, 49, 245–253. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, F.; Ren, Z.; Ren, W.; Yu, L.; Jiang, L.; Ren, B.; Wang, L.; Wang, Z.; Yu, Y.; et al. An Improved Method to Synthesize Nanoscale Graphene Oxide Using Much Less Acid. Mater. Today Phys. 2019, 9, 100097. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cheng, X.; Tian, G.; Zhao, H.; He, L.; Hu, J.; Wu, S.-M.; Dong, Y.; Chang, G.-G.; Lenaerts, S.; et al. Hierarchical CdS/m-TiO2/G ternary photocatalyst for highly active visible light-induced hydrogen production from water splitting with high stability. Nano Energy 2018, 47, 8–17. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Liu, W.; Liang, L.; Wang, S.; Zhang, F.; Shi, H.; Yang, M. A novel approach to fabricate polyacrylate modified graphene oxide for improving the corrosion resistance of epoxy coatings. Colloids Surf. A 2020, 593, 124627. [Google Scholar] [CrossRef]
- Ramezanzadeha, B.; Bahlakehb, G.; Ramezanzadeha, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, H.; Zou, Y.; Zhao, H. Study on self-healing and corrosion resistance behaviors of functionalized carbon dot-intercalated graphene-based waterborne epoxy coating. J. Mater. Sci. Technol. 2021, 67, 226–236. [Google Scholar] [CrossRef]
- Haeri, Z.; Ramezanzadeh, B.; Asghari, M. A novel fabrication of a high performance SiO2-graphene oxide (GO) nanohybrids: Characterization of thermal properties of epoxy nanocomposites filled with SiO2-GO nanohybrids. J. Colloid Interface Sci. 2017, 493, 111–122. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.M. Investigating the effect of SiO2-graphene oxide hybrid as inorganic nanofiller on corrosion protection properties of epoxy coatings. Surf. Coat. Technol. 2017, 311, 282–294. [Google Scholar] [CrossRef]
- Liu, X.; Jie, H.; Liu, R.; Liu, Y.; Li, T.; Lyu, K. Research on the Preparation and Anticorrosion Properties of EP/CeO2-GO Nanocomposite Coating. Polymers 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, R.; Li, T.; Liu, Y.; Liu, L.; Lyu, K.; Shah, S.P. Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water. Polymers 2021, 13, 2072. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide-alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Amudha, A.; Shashikala, H.D.; Rahman, O.S.A.; Keshri, A.K.; Nagaraja, H.S. Effect of graphene oxide loading on plasma sprayed alumina-graphene oxide composites for improved anticorrosive and hydrophobic surface. Surf. Topogr. Metrol. Prop. 2019, 7, 024003. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, L.; Liang, G.; Gu, A. Mechanically durable and self-healing super-hydrophobic coating with hierarchically structured KH570 modified SiO2-decorated aligned carbon nanotube bundles. Chem. Eng. J. 2021, 408, 127263. [Google Scholar] [CrossRef]
- Shi, F.; Xu, J.; Zhang, Z. Study on UV-protection and hydrophobic properties of cotton fabric functionalized by graphene oxide and silane coupling agent. Pigm. Resin Technol. 2019, 48, 237–242. [Google Scholar] [CrossRef]
- Shen, L.; Ying, J.; Tian, G.; Jia, M.-P.; Yang, X.-Y. Ultralong PtPd Alloyed Nanowires Anchored on Graphene for Efficient Methanol Oxidation Reaction. Chem. -Asian J. 2021, 16, 1130–1137. [Google Scholar] [CrossRef]
- Xiao, Y.-X.; Ying, J.; Tian, G.; Yang, X.; Zhang, Y.-X.; Chen, J.-B.; Wang, Y.; Symes, M.D.; Ozoemena, K.I.; Wu, J.; et al. Hierarchically Fractal PtPdCu Sponges and their Directed Mass- and Electron-Transfer Effects. Nano Lett. 2021, 21, 7870–7878. [Google Scholar] [CrossRef]
- O’Hare, L.-A.; Parboo, B.; Leadley, S.R. Development of a methodology for XPS curve-fitting of the Si 2p core level of siloxane materials. Surf. Interface Anal. 2004, 36, 1427–1434. [Google Scholar] [CrossRef]
- Parhizkara, N.; Shahrabia, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhao, L.; Fan, Q.; Zeng, X.; Liu, S.; Pang, W.K.; He, Y.-B.; Guo, Z. Lithium Metal Electrode with Increased Air Stability and Robust Solid Electrolyte Interphase Realized by Silane Coupling Agent Modification. Adv. Mater. 2021, 33, 2008133. [Google Scholar] [CrossRef] [PubMed]
- Pulikkalparambil, H.; Siengchin, S.; Parameswaranpillai, J. Corrosion protective self-healing epoxy resin coatings based on inhibitor and polymeric healing agents encapsulated in organic and inorganic micro and nanocontainers. Nano-Struct. Nano-Objects 2018, 16, 381–395. [Google Scholar] [CrossRef]
- Xiao, Y.-X.; Ying, J.; Tian, G.; Tao, Y.; Wei, H.; Fan, S.-Y.; Sun, Z.-H.; Zou, W.-J.; Hu, J.; Chang, G.-G.; et al. Highly dispersed PtPd on graphitic nanofibers and its heavy d-π effect. Appl. Catal. B 2019, 259, 118080. [Google Scholar] [CrossRef]
- Lee, S.; Noh, J.; Hong, S.; Kim, Y.K.; Jang, J. Dual Stimuli-Responsive Smart Fluid of Graphene Oxide-Coated Iron Oxide/Silica Core/Shell Nanoparticles. Chem. Mater. 2016, 28, 2624–2633. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Corrosion of X52 pipeline steel in a simulated soil solution with coexistence of Desulfovibrio desulfuricans and Pseudomonas aeruginosa bacteria. Corros. Sci. 2020, 173, 108753. [Google Scholar] [CrossRef]
- Yuan, R.; Ju, P.; Wu, Y.; Ji, L.; Li, H.; Chen, L.; Zhou, H.; Chen, J. Silane-grafted graphene oxide improves wear and corrosion resistance of polyimide matrix: Molecular dynamics simulation and experimental analysis. J. Mater Sci. 2019, 54, 11069–11083. [Google Scholar] [CrossRef]








| Element | GO | G-K | G-S | G-S-K | ||||
|---|---|---|---|---|---|---|---|---|
| Weight (%) | Atom (%) | Weight (%) | Atom (%) | Weight (%) | Atom (%) | Weight (%) | Atom (%) | |
| C | 68.20 | 74.08 | 44.39 | 55.26 | 21.75 | 30.96 | 36.91 | 47.77 |
| O | 31.80 | 25.92 | 37.63 | 35.16 | 46.62 | 49.80 | 41.38 | 40.21 |
| Si | - | - | 17.98 | 9.58 | 31.63 | 19.24 | 21.71 | 12.02 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Time/day | 0 (Ω cm2) | 10 (Ω cm2) |
|---|---|---|
| epoxy | 2.5 × 106 | 1.3 × 103 |
| EP-GO | 1.6 × 108 | 3.4 × 104 |
| EP-G-K | 7.0 × 108 | 7.6 × 103 |
| EP-G-S | 1.1 × 109 | 4.5 × 104 |
| EP-G-S-K | 9.7 × 108 | 1.9 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Xiao, Y.; Ying, J. Nano-SiO2 and Silane Coupling Agent Co-Decorated Graphene Oxides with Enhanced Anti-Corrosion Performance of Epoxy Composite Coatings. Int. J. Mol. Sci. 2021, 22, 11087. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222011087
Hu G, Xiao Y, Ying J. Nano-SiO2 and Silane Coupling Agent Co-Decorated Graphene Oxides with Enhanced Anti-Corrosion Performance of Epoxy Composite Coatings. International Journal of Molecular Sciences. 2021; 22(20):11087. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222011087
Chicago/Turabian StyleHu, Guangjie, Yuxuan Xiao, and Jie Ying. 2021. "Nano-SiO2 and Silane Coupling Agent Co-Decorated Graphene Oxides with Enhanced Anti-Corrosion Performance of Epoxy Composite Coatings" International Journal of Molecular Sciences 22, no. 20: 11087. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222011087