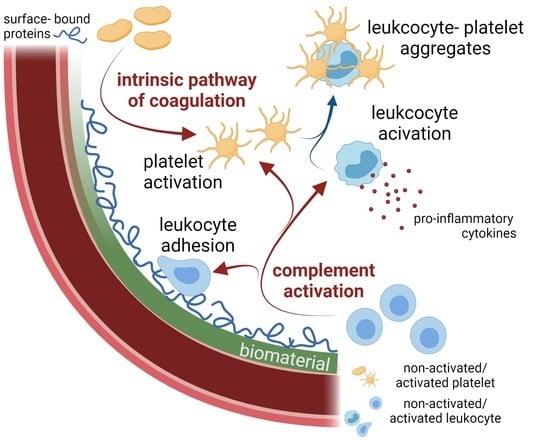
Predicting the In Vivo Performance of Cardiovascular Biomaterials: Current Approaches In Vitro Evaluation of Blood-Biomaterial Interactions
Abstract
:1. Introduction
2. Complement Activation by Biomaterials
3. Complement-Coagulation Interplay
4. Modulation of Complement Activation by Biomaterials
4.1. Surface Chemistry
4.2. Topography
4.3. Surface Roughness and Stiffness
4.4. Immune Modulation Strategies
5. In Vitro Testing
5.1. Protein Adsorption
5.2. Contact System, Coagulation Cascade and Platelet Activation
5.3. Complement and Leukocyte Activation
5.4. Leukocyte-Platelet Aggregates
5.5. Proteomic Approaches
6. Concluding Remarks and Open Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jannasch, M.; Gaetzner, S.; Groeber, F.; Weigel, T.; Walles, H.; Schmitz, T.; Hansmann, J. An in vitro model mimics the contact of biomaterials to blood components and the reaction of surrounding soft tissue. Acta Biomater. 2019, 89, 227–241. [Google Scholar] [CrossRef]
- Andersson, J.; Ekdahl, K.N.; Lambris, J.D.; Nilsson, B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials 2005, 26, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, E.; Lisignoli, G.; Borzi, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, B.; Korsgren, O.; Lambris, J.D.; Ekdahl, K.N. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010, 31, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Reviakine, I.; Jung, F.; Braune, S.; Brash, J.L.; Latour, R.; Gorbet, M.; van Oeveren, W. Stirred, shaken, or stagnant: What goes on at the blood-biomaterial interface. Blood Rev. 2017, 31, 11–21. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Huang, S.; Nilsson, B.; Teramura, Y. Complement inhibition in biomaterial- and biosurface-induced thromboinflammation. Semin. Immunol. 2016, 28, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Alexander, M.R.; Williams, P. Water contact angle is not a good predictor of biological responses to materials. Biointerphases 2017, 12, 02C201. [Google Scholar] [CrossRef] [Green Version]
- Grainger, D.W. All charged up about implanted biomaterials. Nat. Biotechnol. 2013, 31, 507–509. [Google Scholar] [CrossRef]
- Abizaid, A.; Costa, J.R. New Drug-Eluting Stents an Overview on Biodegradable and Polymer-Free Next-Generation Stent Systems. Circ. Cardiovasc. Interv. 2010, 3, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Supriyanto, E.; Murugesan, S.; Balaji, A.; Asokan, M.K. Biomaterials in Cardiovascular Research: Applications and Clinical Implications. Biomed. Res. Int. 2014, 2014, 459465. [Google Scholar] [CrossRef] [Green Version]
- Pacharra, S.; McMahon, S.; Duffy, P.; Basnett, P.; Yu, W.; Seisel, S.; Stervbo, U.; Babel, N.; Roy, I.; Viebahn, R.; et al. Cytocompatibility Evaluation of a Novel Series of PEG-Functionalized Lactide-Caprolactone Copolymer Biomaterials for Cardiovascular Applications. Front. Bioeng. Biotechnol. 2020, 8, 991. [Google Scholar] [CrossRef]
- Fernandez, J.; Etxeberria, A.; Sarasua, J.R. Synthesis, structure and properties of poly(L-lactide-co-epsilon-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Strohbach, A.; Maess, F.; Wulf, K.; Petersen, S.; Grabow, N.; Schmitz, K.P.; Felix, S.B.; Busch, R. The Role of Biodegradable Poly-(L-lactide)-Based Polymers in Blood Cell Activation and Platelet-Monocyte Interaction. Int. J. Mol. Sci. 2021, 22, 6340. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. Complement and coagulation: Strangers or partners in crime? Trends Immunol. 2007, 28, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007, 44, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Ekdahl, K.N.; Larsson, R.; Nilsson, U.R.; Nilsson, B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J. Immunol. 2002, 168, 5786–5791. [Google Scholar] [CrossRef] [Green Version]
- Ekdahl, K.N.; Lambris, J.D.; Elwing, H.; Ricklin, D.; Nilsson, P.H.; Teramura, Y.; Nicholls, I.A.; Nilsson, B. Innate immunity activation on biomaterial surfaces: A mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 2011, 63, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Chen, E.Y.; Liu, W.F. Biomolecular strategies to modulate the macrophage response to implanted materials. J. Mater. Chem. B 2016, 4, 1600–1609. [Google Scholar] [CrossRef]
- Parente, R.; Clark, S.J.; Inforzato, A.; Day, A.J. Complement factor H in host defense and immune evasion. Cell Mol. Life Sci. 2017, 74, 1605–1624. [Google Scholar] [CrossRef]
- Wettero, J.; Askendal, A.; Bengtsson, T.; Tengvall, P. On the binding of complement to solid artificial surfaces in vitro. Biomaterials 2002, 23, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Sperling, C.; Schweiss, R.B.; Streller, U.; Werner, C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials 2005, 26, 6547–6557. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Kitazawa, T.; Hirata, I.; Hirano, Y.; Iwata, H. Complement activation on surfaces carrying amino groups. Biomaterials 2008, 29, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Kutryk, M.J.; Ong, A.T. Coronary-artery stents. N. Engl. J. Med. 2006, 354, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Wijns, W.; Steg, P.G.; Mauri, L.; Kurowski, V.; Parikh, K.; Gao, R.; Bode, C.; Greenwood, J.P.; Lipsic, E.; Alamgir, F.; et al. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur. Heart J. 2014, 35, 2812–2820. [Google Scholar] [CrossRef] [Green Version]
- Kirtane, A.J.; Leon, M.B.; Ball, M.W.; Bajwa, H.S.; Sketch, M.H., Jr.; Coleman, P.S.; Stoler, R.C.; Papadakos, S.; Cutlip, D.E.; Mauri, L.; et al. The “final” 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc. Interv. 2013, 6, 325–333. [Google Scholar] [CrossRef]
- Maeng, M.; Tilsted, H.H.; Jensen, L.O.; Krusell, L.R.; Kaltoft, A.; Kelbaek, H.; Villadsen, A.B.; Ravkilde, J.; Hansen, K.N.; Christiansen, E.H.; et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): A multicentre, open-label, randomised superiority trial. Lancet 2014, 383, 2047–2056. [Google Scholar] [CrossRef]
- Inoue, Y.; Onodera, Y.; Ishihara, K. Preparation of a thick polymer brush layer composed of poly(2-methacryloyloxyethyl phosphorylcholine) by surface-initiated atom transfer radical polymerization and analysis of protein adsorption resistance. Colloids Surf. B Biointerfaces 2016, 141, 507–512. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xue, Y.F.; Chen, T.T.; Huang, D.N.; Wang, Y.X.; Ren, K.F.; Wang, Y.B.; Fu, G.S.; Ji, J. Biodegradable phosphorylcholine copolymer for cardiovascular stent coating. J. Mater. Chem. B 2020, 8, 5361–5368. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Tondera, C.; Hauser, S.; Braune, S.; Gors, J.; Roch, T.; Klopfleisch, R.; Neffe, A.T.; Lendlein, A.; Pietzsch, J.; et al. Immunocompatibility and non-thrombogenicity of gelatin-based hydrogels. Clin. Hemorheol. Microcirc. 2021, 77, 335–350. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.W.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahid, F.; Zhao, X.J.; Jia, S.R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200. [Google Scholar] [CrossRef]
- Apte, G.; Lindenbauer, A.; Schemberg, J.; Rothe, H.; Nguyen, T.H. Controlling Surface-Induced Platelet Activation by Agarose and Gelatin-Based Hydrogel Films. ACS Omega 2021, 6, 10963–10974. [Google Scholar] [CrossRef]
- Fischer, M.; Sperling, C.; Tengvall, P.; Werner, C. The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials 2010, 31, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Horbett, T.A. Fibrinogen adsorption to biomaterials. J. Biomed. Mater. Res. A 2018, 106, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Long, A.T.; Kenne, E.; Jung, R.; Fuchs, T.A.; Renne, T. Contact system revisited: An interface between inflammation, coagulation, and innate immunity. J. Thromb. Haemost. 2016, 14, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Zhuo, R.; Siedlecki, C.A.; Vogler, E.A. Competitive-protein adsorption in contact activation of blood factor XII. Biomaterials 2007, 28, 4355–4369. [Google Scholar] [CrossRef] [Green Version]
- Chiumiento, A.; Lamponi, S.; Barbucci, R. Role of fibrinogen conformation in platelet activation. Biomacromolecules 2007, 8, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Simonovsky, F.I.; Ratner, B.D.; Horbett, T.A. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: A comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J. Biomed. Mater. Res. A 2005, 74, 722–738. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact activation of blood-plasma coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, O.A.; Ekdahl, K.N.; Nilsson, P.H.; Andersson, J.; Magotti, P.; Lambris, J.D.; Nilsson, B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J. Thromb. Haemost. 2008, 6, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Hamad, O.A.; Mitroulis, I.; Fromell, K.; Kozarcanin, H.; Chavakis, T.; Ricklin, D.; Lambris, J.D.; Ekdahl, K.N.; Nilsson, B. Contact activation of C3 enables tethering between activated platelets and polymorphonuclear leukocytes via CD11b/CD18. Thromb. Haemost. 2015, 114, 1207–1217. [Google Scholar] [CrossRef] [Green Version]
- Hamad, O.A.; Nilsson, P.H.; Lasaosa, M.; Ricklin, D.; Lambris, J.D.; Nilsson, B.; Ekdahl, K.N. Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS ONE 2010, 5, e12889. [Google Scholar] [CrossRef] [Green Version]
- Engberg, A.E.; Nilsson, P.H.; Huang, S.; Fromell, K.; Hamad, O.A.; Mollnes, T.E.; Rosengren-Holmberg, J.P.; Sandholm, K.; Teramura, Y.; Nicholls, I.A.; et al. Prediction of inflammatory responses induced by biomaterials in contact with human blood using protein fingerprint from plasma. Biomaterials 2015, 36, 55–65. [Google Scholar] [CrossRef]
- Huang, S.; Engberg, A.E.; Jonsson, N.; Sandholm, K.; Nicholls, I.A.; Mollnes, T.E.; Fromell, K.; Nilsson, B.; Ekdahl, K.N. Reciprocal relationship between contact and complement system activation on artificial polymers exposed to whole human blood. Biomaterials 2016, 77, 111–119. [Google Scholar] [CrossRef]
- Arkowski, J.; Obremska, M.; Kedzierski, K.; Slawuta, A.; Wawrzynska, M. Applications for graphene and its derivatives in medical devices: Current knowledge and future applications. Adv. Clin. Exp. Med. 2020, 29, 1497–1504. [Google Scholar] [CrossRef]
- ElSawy, A.M.; Attia, N.F.; Mohamed, H.I.; Mohsen, M.; Talaat, M.H. Innovative coating based on graphene and their decorated nanoparticles for medical stent applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 708–715. [Google Scholar] [CrossRef]
- Ge, S.; Xi, Y.; Du, R.; Ren, Y.; Xu, Z.; Tan, Y.; Wang, Y.; Yin, T.; Wang, G. Inhibition of in-stent restenosis after graphene oxide double-layer drug coating with good biocompatibility. Regen. Biomater. 2019, 6, 299–309. [Google Scholar] [CrossRef]
- Yang, M.C.; Tsou, H.M.; Hsiao, Y.S.; Cheng, Y.W.; Liu, C.C.; Huang, L.Y.; Peng, X.Y.; Liu, T.Y.; Yung, M.C.; Hsu, C.C. Electrochemical Polymerization of PEDOT-Graphene Oxide-Heparin Composite Coating for Anti-fouling and Anti-clotting of Cardiovascular Stents. Polymers 2019, 11, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Cheng, Y.; Wang, P.; Chen, K.; Chen, Z.; Liu, X.; Fu, X.; Wang, K.; Liu, K.; Liu, Z.; et al. Enhanced Hemocompatibility of a Direct Chemical Vapor Deposition-Derived Graphene Film. ACS Appl. Mater. Interfaces 2021, 13, 4835–4843. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., 2nd; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, P.; Neumann, B.; Haag, H.; Li, M.; Xu, Z.; Zhou, C.; Scheideler, L.; Wendel, H.P.; Zhang, H.; et al. Chandler-Loop surveyed blood compatibility and dynamic blood triggered degradation behavior of Zn-4Cu alloy and Zn. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111594. [Google Scholar] [CrossRef]
- Dinnes, D.L.; Marcal, H.; Mahler, S.M.; Santerre, J.P.; Labow, R.S. Material surfaces affect the protein expression patterns of human macrophages: A proteomics approach. J. Biomed. Mater. Res. A 2007, 80, 895–908. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Kumar, N.; Parajuli, O.; Gupta, A.; Hahm, J.I. Elucidation of protein adsorption behavior on polymeric surfaces: Toward high-density, high-payload protein templates. Langmuir 2008, 24, 2688–2694. [Google Scholar] [CrossRef]
- Ouberai, M.M.; Xu, K.; Welland, M.E. Effect of the interplay between protein and surface on the properties of adsorbed protein layers. Biomaterials 2014, 35, 6157–6163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Ravensbergen, K.; Alabanza, A.; Soldin, D.; Hahm, J.I. Distinct adsorption configurations and self-assembly characteristics of fibrinogen on chemically uniform and alternating surfaces including block copolymer nanodomains. ACS Nano 2014, 8, 5257–5269. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Maitz, M.F.; Werner, C. Coatings for biomaterials to improve hemocompatibility. In Hemocompatibility of Biomaterials for Clinical Application; Siedlecki, C.A., Ed.; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Chang, D.T.; Colton, E.; Matsuda, T.; Anderson, J.M. Lymphocyte adhesion and interactions with biomaterial adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2009, 91, 1210–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, S.; Bhattacharyya, D.; Padukudru, C.; Timmons, R.B.; Tang, L. Surface chemistry influences implant-mediated host tissue responses. J. Biomed. Mater. Res. A 2008, 86, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Zou, L.; Bhattacharyya, D.; Timmons, R.B.; Tang, L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir 2008, 24, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- Wise, S.G.; Waterhouse, A.; Kondyurin, A.; Bilek, M.M.; Weiss, A.S. Plasma-based biofunctionalization of vascular implants. Nanomedicine 2012, 7, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, B.; Latour, R.A. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials 2010, 31, 832–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Sperling, C.; Werner, C. Synergistic effect of hydrophobic and anionic surface groups triggers blood coagulation in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Christo, S.N.; Bachhuka, A.; Diener, K.R.; Mierczynska, A.; Hayball, J.D.; Vasilev, K. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. Adv. Healthc. Mater. 2016, 5, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podesta, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef] [Green Version]
- Ercan, B.; Khang, D.; Carpenter, J.; Webster, T.J. Using mathematical models to understand the effect of nanoscale roughness on protein adsorption for improving medical devices. Int. J. Nanomedicine 2013, 8 (Suppl. S1), 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.C.; Haberstroh, K.M.; Webster, T.J. Mechanism(s) of increased vascular cell adhesion on nanostructured poly(lactic-co-glycolic acid) films. J. Biomed. Mater. Res. A 2005, 73, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; Shah, A.; Enciso, A.E.; Chan, K.C.; Kaczmarczyk, J.A.; Blonder, J.; Simanek, E.E.; Dobrovolskaia, M.A. Nanoparticle physicochemical properties determine the activation of intracellular complement. Nanomedicine 2019, 17, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Hulander, M.; Lundgren, A.; Faxalv, L.; Lindahl, T.L.; Palmquist, A.; Berglin, M.; Elwing, H. Gradients in surface nanotopography used to study platelet adhesion and activation. Colloids Surf. B Biointerfaces 2013, 110, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, F. Decoy nanoparticles bearing native C5a receptors as a new approach to inhibit complement-mediated neutrophil activation. Acta Biomater. 2019, 99, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M.; Wiacek, A.E.; Ladniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface Roughness and Substrate Stiffness Synergize To Drive Cellular Mechanoresponse. Nano Lett. 2020, 20, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, M.; Kiesswetter, V.; Slottosch, I.; Awad, G.; Paunel-Gorgulu, A.; Varghese, S.; Klopfleisch, M.; Kupitz, D.; Klemm, D.; Nietzsche, S.; et al. In vitro hemo- and cytocompatibility of bacterial nanocelluose small diameter vascular grafts: Impact of fabrication and surface characteristics. PLoS ONE 2020, 15, e0235168. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Kiick, K.L.; Akins, R.E. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 2021, 122, 220–235. [Google Scholar] [CrossRef]
- Vyner, M.C.; Amsden, B.G. Polymer chain flexibility-induced differences in fetuin A adsorption and its implications on cell attachment and proliferation. Acta Biomater. 2016, 31, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Nilsson, P.H.; Ekdahl, K.N.; Magnusson, P.U.; Qu, H.; Iwata, H.; Ricklin, D.; Hong, J.; Lambris, J.D.; Nilsson, B.; Teramura, Y. Autoregulation of thromboinflammation on biomaterial surfaces by a multicomponent therapeutic coating. Biomaterials 2013, 34, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abaricia, J.O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Jain, N.; Moeller, J.; Vogel, V. Mechanobiology of Macrophages: How Physical Factors Coregulate Macrophage Plasticity and Phagocytosis. Annu. Rev. Biomed. Eng. 2019, 21, 267–297. [Google Scholar] [CrossRef]
- Witherel, C.E.; Graney, P.L.; Spiller, K.L. In Vitro Model of Macrophage-Biomaterial Interactions. Methods Mol. Biol. 2018, 1758, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.S.; Zhao, G.L.; Liu, Q.; Jiang, S.C.; Wang, Y.; Zhang, D.M. Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. J. Inflamm. 2015, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2007, 83, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Clark, N.M.; Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 2018, 182, 202–215. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef] [Green Version]
- Kourtzelis, I.; Markiewski, M.M.; Doumas, M.; Rafail, S.; Kambas, K.; Mitroulis, I.; Panagoutsos, S.; Passadakis, P.; Vargemezis, V.; Magotti, P.; et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood 2010, 116, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramyan, T.M.; Hyde-Volpe, D.L.; Stuart, S.J.; Latour, R.A. Application of advanced sampling and analysis methods to predict the structure of adsorbed protein on a material surface. Biointerphases 2017, 12, 02D409. [Google Scholar] [CrossRef]
- Braune, S.; Gross, M.; Walter, M.; Zhou, S.; Dietze, S.; Rutschow, S.; Lendlein, A.; Tschope, C.; Jung, F. Adhesion and activation of platelets from subjects with coronary artery disease and apparently healthy individuals on biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 210–217. [Google Scholar] [CrossRef]
- Fromell, K.; Yang, Y.; Nilsson Ekdahl, K.; Nilsson, B.; Berglin, M.; Elwing, H. Absence of conformational change in complement factor 3 and factor XII adsorbed to acrylate polymers is related to a high degree of polymer backbone flexibility. Biointerphases 2017, 12, 02D417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peerschke, E.I.; Yin, W.; Ghebrehiwet, B. Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol. Immunol. 2010, 47, 2170–2175. [Google Scholar] [CrossRef] [Green Version]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, L.; Li, D.; Tang, Z.; Wang, Y.; Chen, G.; Chen, H.; Brash, J.L. Blood compatible materials: State of the art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef]
- Reinthaler, M.; Jung, F.; Landmesser, U.; Lendlein, A. Trend to move from permanent metals to degradable, multifunctional polymer or metallic implants in the example of coronary stents. Expert Rev. Med. Devices 2016, 13, 1001–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernagallo, S.; Tura, O.; Wu, M.; Samuel, K.; Diaz-Mochon, J.J.; Hansen, A.; Zhang, R.; Jackson, M.; Padfield, G.J.; Hadoke, P.W.; et al. Novel biopolymers to enhance endothelialisation of intra-vascular devices. Adv. Healthc. Mater. 2012, 1, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Latour, R.A.; Reinthaler, M.; Landmesser, U.; Lendlein, A.; Jung, F. In Vitro Thrombogenicity Testing of Biomaterials. Adv. Healthc. Mater. 2019, 8, e1900527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization. Biological Evaluation of Medical Devices. 2017. Available online: https://www.iso.org/standard/63448.html (accessed on 1 July 2021).
- Braune, S.; Grunze, M.; Straub, A.; Jung, F. Are there sufficient standards for the in vitro hemocompatibility testing of biomaterials? Biointerphases 2013, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, F.; Braune, S.; Lendlein, A. Haemocompatibility testing of biomaterials using human platelets. Clin. Hemorheol. Microcirc. 2013, 53, 97–115. [Google Scholar] [CrossRef]
- Henno, L.T.; Storjord, M.; Christiansen, D.; Bergseth, G.; Ludviksen, J.K.; Fure, H.; Barene, S.; Waage-Nielsen, E.; Mollnes, T.E.; Brekke, O.L. Effect of of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines—Consequences for defining reference values in healthy humans. Cytokine 2017, 97, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.H.H.; Pieper, I.L.; Robinson, C.R.; Friedmann, Y.; Kanamarlapudi, V.; Thornton, C.A. Shear Stress-Induced Total Blood Trauma in Multiple Species. Artif. Organs 2017, 41, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hofferbert, B.V.; Koo, G.; Malinauskas, R.A. In vitro shear stress-induced platelet activation: Sensitivity of human and bovine blood. Artif. Organs 2013, 37, 894–903. [Google Scholar] [CrossRef]
- Braune, S.; Lendlein, A.; Jung, F. Developing standards and test protocols for testing the hemocompatibility of biomaterials. In Hemocompatibility of Biomaterials for Clinical Applications; Siedlecki, C.A., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 51–76. [Google Scholar]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Xu, L.C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vroman, L. The life of an artificial device in contact with blood: Initial events and their effect on its final state. Bull. N. Y. Acad. Med. 1988, 64, 352–357. [Google Scholar]
- Kim, J. Systematic approach to characterize the dynamics of protein adsorption on the surface of biomaterials using proteomics. Colloids Surf. B Biointerfaces 2020, 188, 110756. [Google Scholar] [CrossRef]
- Hirsh, S.L.; McKenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M.M. The Vroman effect: Competitive protein exchange with dynamic multilayer protein aggregates. Colloids Surf. B Biointerfaces 2013, 103, 395–404. [Google Scholar] [CrossRef]
- Thyparambil, A.A.; Wei, Y.; Latour, R.A. Experimental characterization of adsorbed protein orientation, conformation, and bioactivity. Biointerphases 2015, 10, 019002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaraman, B.; Latour, R.A. The adherence of platelets to adsorbed albumin by receptor-mediated recognition of binding sites exposed by adsorption-induced unfolding. Biomaterials 2010, 31, 1036–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekdahl, K.N.; Soveri, I.; Hilborn, J.; Fellstrom, B.; Nilsson, B. Cardiovascular disease in haemodialysis: Role of the intravascular innate immune system. Nat. Rev. Nephrol. 2017, 13, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.W.; Xu, L.C.; Vogler, E.A.; Siedlecki, C.A. Surface dependent contact activation of factor XII and blood plasma coagulation induced by mixed thiol surfaces. Biointerphases 2017, 12, 02D410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clauser, J.C.; Maas, J.; Arens, J.; Schmitz-Rode, T.; Steinseifer, U.; Berkels, B. Hemocompatibility Evaluation of Biomaterials-The Crucial Impact of Analyzed Area. ACS Biomater. Sci. Eng. 2021, 7, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Eichinger, C.D.; Hlady, V. Effects of upstream shear forces on priming of platelets for downstream adhesion and activation. Acta Biomater. 2018, 73, 228–235. [Google Scholar] [CrossRef]
- Slepian, M.J.; Sheriff, J.; Hutchinson, M.; Tran, P.; Bajaj, N.; Garcia, J.G.N.; Scott Saavedra, S.; Bluestein, D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J. Biomech. 2017, 50, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, G.E.; Blok, S.L.; van Oeveren, W. In vitro blood flow model with physiological wall shear stress for hemocompatibility testing-An example of coronary stent testing. Biointerphases 2016, 11, 031004. [Google Scholar] [CrossRef]
- Affeld, K.; Schaller, J.; Wolken, T.; Krabatsch, T.; Kertzscher, U. Role of flow for the deposition of platelets. Biointerphases 2016, 11, 029804. [Google Scholar] [CrossRef]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Int. J. Polym. Sci. 2015, 2015, 782653. [Google Scholar] [CrossRef] [Green Version]
- Busch, R.; Strohbach, A.; Rethfeldt, S.; Walz, S.; Busch, M.; Petersen, S.; Felix, S.; Sternberg, K. New stent surface materials: The impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets. Acta Biomater. 2014, 10, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, B.M.; Porada, C.D.; Atala, A.; Almeida-Porada, G. Microfluidic devices for studying coagulation biology. Semin. Cell Dev. Biol. 2021, 112, 1–7. [Google Scholar] [CrossRef]
- Du, G.; Fang, Q.; den Toonder, J.M. Microfluidics for cell-based high throughput screening platforms—A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Jamiolkowski, M.A.; Hartung, M.C.; Malinauskas, R.A.; Lu, Q. An In Vitro Blood Flow Loop System for Evaluating the Thrombogenicity of Medical Devices and Biomaterials. ASAIO J. 2020, 66, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Sperling, C.; Maitz, M.F.; Steinseifer, U.; Clauser, J.; Hiebl, B.; Krajewski, S.; Wendel, H.P.; Jung, F. Evaluation of platelet adhesion and activation on polymers: Round-robin study to assess inter-center variability. Colloids Surf. B Biointerfaces 2017, 158, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavelayudam, S.K.; Rubenstein, D.A.; Yin, W. Effects of physiologically relevant dynamic shear stress on platelet complement activation. Platelets 2011, 22, 602–610. [Google Scholar] [CrossRef]
- Urbich, C.; Fritzenwanger, M.; Zeiher, A.M.; Dimmeler, S. Laminar shear stress upregulates the complement-inhibitory protein clusterin: A novel potent defense mechanism against complement-induced endothelial cell activation. Circulation 2000, 101, 352–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinderlerer, A.R.; Ali, F.; Johns, M.; Lidington, E.A.; Leung, V.; Boyle, J.J.; Hamdulay, S.S.; Evans, P.C.; Haskard, D.O.; Mason, J.C. KLF2-dependent, shear stress-induced expression of CD59: A novel cytoprotective mechanism against complement-mediated injury in the vasculature. J. Biol. Chem. 2008, 283, 14636–14644. [Google Scholar] [CrossRef] [Green Version]
- Bongrazio, M.; Pries, A.R.; Zakrzewicz, A. The endothelium as physiological source of properdin: Role of wall shear stress. Mol. Immunol. 2003, 39, 669–675. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333. [Google Scholar] [CrossRef] [Green Version]
- De Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Gaertner, F.; Massberg, S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin. Immunol. 2016, 28, 561–569. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef] [Green Version]
- Alcaide, P.; Auerbach, S.; Luscinskas, F.W. Neutrophil recruitment under shear flow: It’s all about endothelial cell rings and gaps. Microcirculation 2009, 16, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Gorbet, M. The effect of shear on in vitro platelet and leukocyte material-induced activation. J. Biomater. Appl. 2013, 28, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Polasky, C.; Wallesch, M.; Loyal, K.; Pries, R.; Wollenberg, B. Measurement of leukocyte-platelet aggregates (LPA) by FACS: A comparative analysis. Platelets 2021, 32, 209–214. [Google Scholar] [CrossRef]
- Busch, G.; Steppich, B.; Sibbing, D.; Braun, S.L.; Stein, A.; Groha, P.; Schomig, A.; Kastrati, A.; von Beckerath, N.; Ott, I. Bivalirudin reduces platelet and monocyte activation after elective percutaneous coronary intervention. Thromb. Haemost. 2009, 101, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.H.; Yuan, S.S.; Chung, T.W.; Jong, S.B.; Lu, C.Y.; Tsai, W.C.; Chen, W.C.; Lin, P.C.; Chiang, P.W.; Tyan, Y.C. Characterization of silk fibroin modified surface: A proteomic view of cellular response proteins induced by biomaterials. Biomed. Res. Int. 2014, 2014, 209469. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Kulak, N.A.; Pichler, G.; Holdt, L.M.; Teupser, D.; Mann, M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milleret, V.; Buzzi, S.; Gehrig, P.; Ziogas, A.; Grossmann, J.; Schilcher, K.; Zinkernagel, A.S.; Zucker, A.; Ehrbar, M. Protein adsorption steers blood contact activation on engineered cobalt chromium alloy oxide layers. Acta Biomater. 2015, 24, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Scott, E.A.; Elbert, D.L. Proteomic analysis of protein adsorption: Serum amyloid P adsorbs to materials and promotes leukocyte adhesion. J. Biomed. Mater. Res. A 2005, 75, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schmidt, D.R.; Joyce, E.J.; Kao, W.J. Application of MS-based proteomics to study serum protein adsorption/absorption and complement C3 activation on poly(ethylene glycol) hydrogels. J. Biomater. Sci. Polym. Ed. 2011, 22, 1343–1362. [Google Scholar] [CrossRef] [Green Version]
- Swartzlander, M.D.; Barnes, C.A.; Blakney, A.K.; Kaar, J.L.; Kyriakides, T.R.; Bryant, S.J. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 2015, 41, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndumiso, M.; Buchtova, N.; Husselmann, L.; Mohamed, G.; Klein, A.; Aucamp, M.; Canevet, D.; D’Souza, S.; Maphasa, R.E.; Boury, F.; et al. Comparative whole corona fingerprinting and protein adsorption thermodynamics of PLGA and PCL nanoparticles in human serum. Colloids Surf. B Biointerfaces 2020, 188, 110816. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Lee, S.; Stone, L.S.; Cerruti, M. Protein Adsorption on Surfaces Functionalized with COOH Groups Promotes Anti-inflammatory Macrophage Responses. ACS Appl. Mater. Interfaces 2021, 13, 7021–7036. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohbach, A.; Busch, R. Predicting the In Vivo Performance of Cardiovascular Biomaterials: Current Approaches In Vitro Evaluation of Blood-Biomaterial Interactions. Int. J. Mol. Sci. 2021, 22, 11390. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222111390
Strohbach A, Busch R. Predicting the In Vivo Performance of Cardiovascular Biomaterials: Current Approaches In Vitro Evaluation of Blood-Biomaterial Interactions. International Journal of Molecular Sciences. 2021; 22(21):11390. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222111390
Chicago/Turabian StyleStrohbach, Anne, and Raila Busch. 2021. "Predicting the In Vivo Performance of Cardiovascular Biomaterials: Current Approaches In Vitro Evaluation of Blood-Biomaterial Interactions" International Journal of Molecular Sciences 22, no. 21: 11390. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222111390