Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet
Abstract
:1. Introduction
2. Results
2.1. Toxicity of Gas Plasma Treatment in Skin Cancer Cells In Vitro
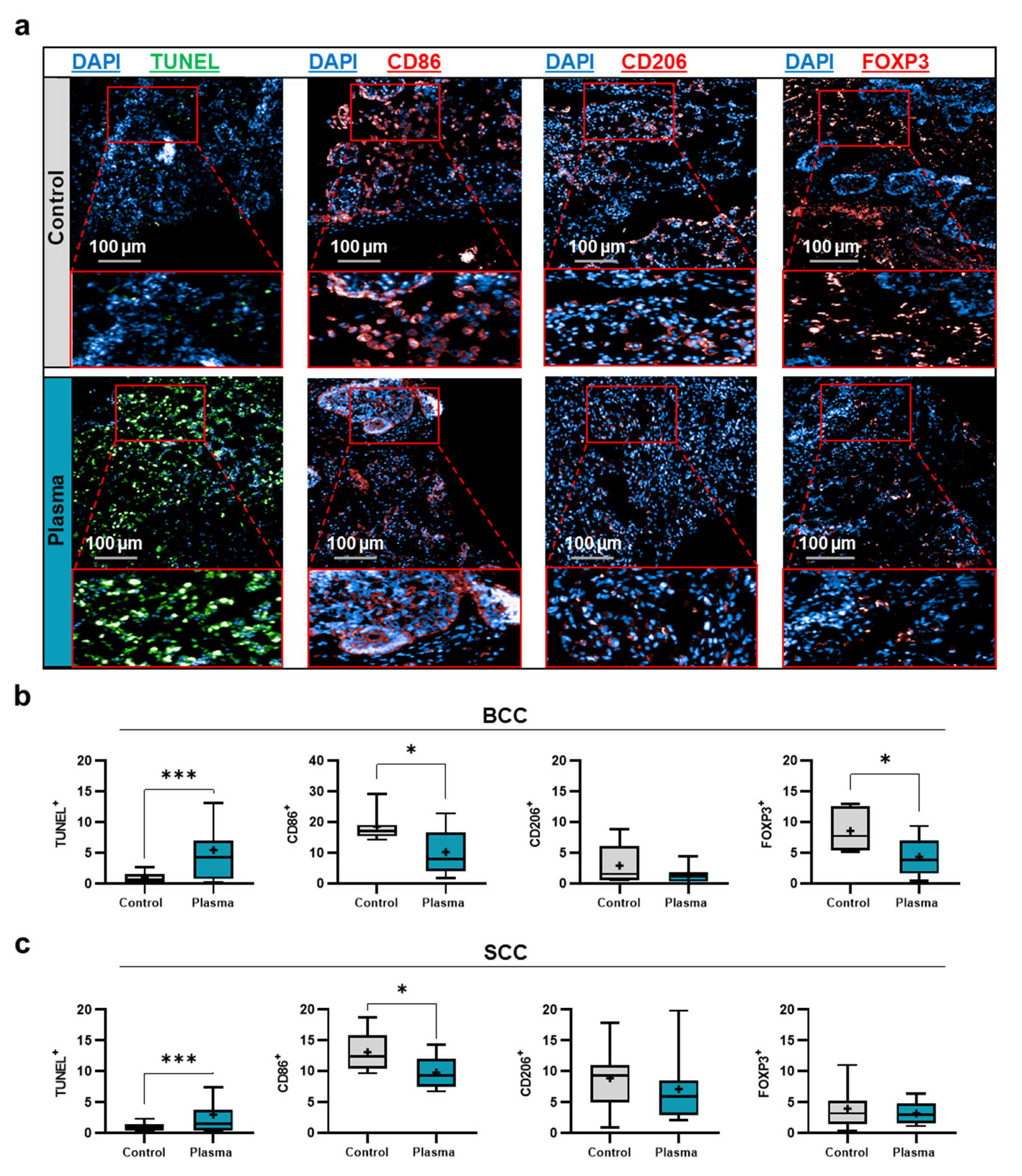
2.2. Toxicity and Immunomodulation of Gas Plasma-Treated Skin Cancer Biopsies
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Primary Skin Tumor Material
4.3. Gas Plasma Exposure
4.4. Metabolic Activity
4.5. Apoptosis Detection
4.6. Flow Cytometry
4.7. Supernatant Analysis
4.8. Tissue Immunofluorescence Analysis
4.9. Reactive Species Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C.J.B. Non melanoma skin cancer pathogenesis overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, M.; Semmler, M.L.; Bernhardt, T.; Fischer, T.; Kakkassery, V.; Ramer, R.; Hein, M.; Bekeschus, S.; Langer, P.; Hinz, B.; et al. Small molecules in the treatment of squamous cell carcinomas: Focus on indirubins. Cancers (Basel) 2021, 13, 1770. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Derm. Pr. Concept. 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour microenvironment in skin carcinogenesis. Tumor Microenviron. Organs 2020, 1226, 123–142. [Google Scholar]
- Ahmed, F.; Haass, N.K. Microenvironment-driven dynamic heterogeneity and phenotypic plasticity as a mechanism of melanoma therapy resistance. Front. Oncol. 2018, 8, 173. [Google Scholar] [CrossRef]
- Muppalla, J.N.K.; MuDDaNa, K.; Dorankula, S.P.R.; Thokala, M.R.; Pasupula, A.P. Microenvironment–a role in tumour progression and prognosis. J. Clin. Diagn. Res. JCDR 2013, 7, 2096. [Google Scholar]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Liebelt, G.; Menz, J.; Berner, J.; Sagwal, S.K.; Wende, K.; Weltmann, K.D.; Boeckmann, L.; von Woedtke, T.; Metelmann, H.R.; et al. Tumor cell metabolism correlates with resistance to gas plasma treatment: The evaluation of three dogmas. Free Radic. Biol. Med. 2021, 167, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Gay-Mimbrera, J.; Garcia, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; Garcia-Nieto, A.V.; Ruano, J. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Wang, Q.; Malyavko, A.; Zolotukhin, D.B.; Adhikari, M.; Sherman, J.H.; Keidar, M. The anti-glioblastoma effect of cold atmospheric plasma treatment: Physical pathway v.s. Chemical pathway. Sci. Rep. 2020, 10, 11788. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination therapy of cold atmospheric plasma (cap) with temozolomide in the treatment of u87mg glioblastoma cells. Sci. Rep. 2020, 10, 16495. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of direct and indirect cold atmospheric-pressure plasma methods in the b16f10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasse, S.; Meder, T.; Freund, E.; von Woedtke, T.; Bekeschus, S. Plasma treatment limits human melanoma spheroid growth and metastasis independent of the ambient gas composition. Cancers (Basel) 2020, 12, 2570. [Google Scholar] [CrossRef]
- Adil, B.H.; Al-Shammari, A.M.; Murbat, H.H. Breast cancer treatment using cold atmospheric plasma generated by the fe-dbd scheme. Clin. Plas. Med. 2020, 19, 100103. [Google Scholar] [CrossRef]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef] [PubMed]
- Terefinko, D.; Dzimitrowicz, A.; Bielawska-Pohl, A.; Klimczak, A.; Pohl, P.; Jamroz, P. The influence of cold atmospheric pressure plasma-treated media on the cell viability, motility, and induction of apoptosis in human non-metastatic (mcf7) and metastatic (mda-mb-231) breast cancer cell lines. Int. J. Mol. Sci. 2021, 22, 3855. [Google Scholar] [CrossRef]
- Schneider, C.; Arndt, S.; Zimmermann, J.L.; Li, Y.; Karrer, S.; Bosserhoff, A.K. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol. Chem. 2018, 400, 111–122. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.D.; Ostrikov, K.K. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by nox2–ask1 apoptosis pathways and oxidative stress is mitigated by srx–nrf2 anti-oxidant system. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2827–2837. [Google Scholar] [CrossRef]
- Chauvin, J.; Judee, F.; Merbahi, N.; Vicendo, P. Effects of plasma activated medium on head and neck fadu cancerous cells: Comparison of 3d and 2d response. Anticancer. Agents Med. Chem. 2018, 18, 776–783. [Google Scholar] [CrossRef]
- Hasse, S.; Seebauer, C.; Wende, K.; Schmidt, A.; Metelmann, H.-R.; von Woedtke, T.; Bekeschus, S. Cold argon plasma as adjuvant tumour therapy on progressive head and neck cancer: A preclinical study. Appl. Sci. 2019, 9, 2061. [Google Scholar] [CrossRef] [Green Version]
- Golubitskaya, E.A.; Troitskaya, O.S.; Yelak, E.V.; Gugin, P.P.; Richter, V.A.; Schweigert, I.V.; Zakrevsky, D.E.; Koval, O.A. Cold physical plasma decreases the viability of lung adenocarcinoma cells. Acta Nat. 2019, 11, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. Ros from physical plasmas: Redox chemistry for biomedical therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of short-lived reactive species in plasma-air-water systems: The dos and the do nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.K. Cold atmospheric plasma causes a calcium influx in melanoma cells triggering cap-induced senescence. Sci. Rep. 2018, 8, 10048. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.-T. Ros-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid. Med. Cell Longev. 2020, 2020, 5047987. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers (Basel) 2019, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Wende, K.; von Woedtke, T.; Weltmann, K.D.; Bekeschus, S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biol. Chem. 2018, 400, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Sardella, E.; Veronico, V.; Gristina, R.; Grossi, L.; Cosmai, S.; Striccoli, M.; Buttiglione, M.; Fracassi, F.; Favia, P. Plasma treated water solutions in cancer treatments: The contrasting role of rns. Antioxidants (Basel) 2021, 10, 605. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ros-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Joh, H.M.; Chung, T.H. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl. Phys. Lett. 2013, 103, 153705. [Google Scholar] [CrossRef]
- Bundscherer, L.; Bekeschus, S.; Tresp, H.; Hasse, S.; Reuter, S.; Weltmann, K.-D.; Lindequist, U.; Masur, K. Viability of human blood leukocytes compared with their respective cell lines after plasma treatment. Plasma Med. 2013, 3, 71–80. [Google Scholar] [CrossRef]
- Girard, P.M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of h2o2 and no2 in cell death induced by cold atmospheric he plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.; Jangra, A.; Choi, S.A.; Choi, E.H.; Han, I. Non-thermal atmospheric pressure bio-compatible plasma stimulates apoptosis via p38/mapk mechanism in u87 malignant glioblastoma. Cancers (Basel) 2020, 12, 245. [Google Scholar] [CrossRef] [Green Version]
- Gherardi, M.; Turrini, E.; Laurita, R.; De Gianni, E.; Ferruzzi, L.; Liguori, A.; Stancampiano, A.; Colombo, V.; Fimognari, C. Atmospheric non-equilibrium plasma promotes cell death and cell-cycle arrest in a lymphoma cell line. Plasma Process. Polym. 2015, 12, 1354–1363. [Google Scholar] [CrossRef]
- Adhikari, M.; Kaushik, N.; Ghimire, B.; Adhikari, B.; Baboota, S.; Al-Khedhairy, A.A.; Wahab, R.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion synergistically inhibits human melanoma tumorigenesis via targeting hgf/c-met downstream pathway. Cell Commun. Signal. 2019, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-g(1) arrest via the atm/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schutz, C.S.; Niessner, F.; Wende, K.; Weltmann, K.D.; Gelbrich, N.; von Woedtke, T.; Schmidt, A.; Stope, M.B. Elevated h2ax phosphorylation observed with kinpen plasma treatment is not caused by ros-mediated DNA damage but is the consequence of apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 8535163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, E.; Spadola, C.; Schmidt, A.; Privat-Maldonado, A.; Bogaerts, A.; von Woedtke, T.; Weltmann, K.-D.; Heidecke, C.-D.; Partecke, L.-I.; Käding, A.; et al. Risk evaluation of emt and inflammation in metastatic pancreatic cancer cells following plasma treatment. Front. Phys. 2020, 8, 569618. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Diedrich, S.; Pati, O.; Freund, E.; Flieger, R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Cold physical plasma selectively elicits apoptosis in murine pancreatic cancer cells in vitro and in ovo. Anticancer. Res. 2018, 38, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Guo, B.; Chen, H.; Xu, D.; Kong, M.G. The antitumor effects of plasma-activated saline on muscle-invasive bladder cancer cells in vitro and in vivo demonstrate its feasibility as a potential therapeutic approach. Cancers (Basel) 2021, 13, 1042. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Craniomaxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Flörcken, A.; Johannsen, M.; Nguyen-Hoai, T.; Gerhardt, A.; Miller, K.; Dörken, B.; Pezzutto, A.; Westermann, J.; Jöhrens, K. Immunomodulatory molecules in renal cell cancer: Cd80 and cd86 are expressed on tumor cells. Int. J. Clin. Exp. Pathol. 2017, 10, 1443–1454. [Google Scholar]
- Harnack, U.; Johnen, H.; Pecher, G. Natural killer cell line yt exerts cytotoxicity against cd86+ myeloma cells. Anticancer. Res. 2011, 31, 475–479. [Google Scholar] [PubMed]
- Wollenberg, B.; Zeidler, R.; Lebeau, A.; Mack, B.; Lang, S. Lack of b7.1 and b7.2 on head and neck cancer cells and possible significance for gene therapy. Int. J. Mol. Med. 1998, 2, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Tian, W.; He, Y.; Li, J.; He, C.; Li, Y.; Liu, N.; Li, J. Integrated analysis reveals prognostic value and immune correlates of cd86 expression in lower grade glioma. Front. Oncol. 2021, 11, 654350. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Ma, L.; Liu, L.; Zhao, G.; Zhang, S.; Dong, L.; Xue, R.; Chen, S. Cd86(+)/cd206(+), diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int. J. Mol. Sci. 2016, 17, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laoui, D.; Movahedi, K.; Van Overmeire, E.; Van den Bossche, J.; Schouppe, E.; Mommer, C.; Nikolaou, A.; Morias, Y.; De Baetselier, P.; Van Ginderachter, J.A. Tumor-associated macrophages in breast cancer: Distinct subsets, distinct functions. Int. J. Dev. Biol. 2011, 55, 861–867. [Google Scholar] [CrossRef]
- Tjiu, J.W.; Chen, J.S.; Shun, C.T.; Lin, S.J.; Liao, Y.H.; Chu, C.Y.; Tsai, T.F.; Chiu, H.C.; Dai, Y.S.; Inoue, H.; et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J. Investig. Dermatol. 2009, 129, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Epstein, C.B.; Banskota, S.; Issner, R.; Kim, Y.; Bernstein, B.E.; Pinello, L.; Asgari, M.M. Epigenetic alterations in keratinocyte carcinoma. J. Investig. Dermatol. 2021, 141, 1207–1218. [Google Scholar] [CrossRef]
- Kaporis, H.G.; Guttman-Yassky, E.; Lowes, M.A.; Haider, A.S.; Fuentes-Duculan, J.; Darabi, K.; Whynot-Ertelt, J.; Khatcherian, A.; Cardinale, I.; Novitskaya, I.; et al. Human basal cell carcinoma is associated with foxp3+ t cells in a th2 dominant microenvironment. J. Investig. Dermatol. 2007, 127, 2391–2398. [Google Scholar] [CrossRef] [Green Version]
- Bridge, J.A.; Lee, J.C.; Daud, A.; Wells, J.W.; Bluestone, J.A. Cytokines, chemokines, and other biomarkers of response for checkpoint inhibitor therapy in skin cancer. Front. Med. (Lausanne) 2018, 5, 351. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T.; Crivellato, E. The r.role of mast cells in human skin cancers. Clin. Exp. Med. 2021, 21, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Granata, F.; Borriello, F.; Marone, G. Controversial role of mast cells in skin cancers. Exp. Dermatol. 2017, 26, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Qin, J.; Zhong, L.; Gong, L.; Zhang, B.; Zhang, Y.; Gao, W.Q. Ccl5-mediated th2 immune polarization promotes metastasis in luminal breast cancer. Cancer Res. 2015, 75, 4312–4321. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. Ccl5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating beta-catenin/stat3 signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef]
- Sun, K.; Gong, C.; Peng, H.; Fang, H.; Zhou, J.; Li, J.; Chen, S.; Zheng, H. High ccl5 expression is associated with osteosarcoma metastasis and poor prognosis of patients with osteosarcoma. Mol. Med. Rep. 2017, 16, 6953–6957. [Google Scholar] [CrossRef] [Green Version]
- Aldinucci, D.; Colombatti, A. The inflammatory chemokine ccl5 and cancer progression. Mediat. Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, C.; Orlandi, A.; Costanza, G.; Di Stefani, A.; Piccioni, A.; Di Cesare, A.; Chiricozzi, A.; Ferlosio, A.; Peris, K.; Fargnoli, M.C. Expression of il-23/th17-related cytokines in basal cell carcinoma and in the response to medical treatments. PLoS ONE 2017, 12, e0183415. [Google Scholar] [CrossRef]
- Lowes, M.A.; Bishop, G.A.; Cooke, B.E.; Barnetson, R.S.; Halliday, G.M. Keratoacanthomas have an immunosuppressive cytokine environment of increased il-10 and decreased gm-csf compared to squamous cell carcinomas. Br. J. Cancer 1999, 80, 1501–1505. [Google Scholar] [CrossRef] [Green Version]
- Kang, A.; Zhao, D.; Yeh, J.J.; Lee, D.J. Updates on immunotherapy for the treatment of skin cancer. Curr. Dermatol. Rep. 2018, 7, 311–320. [Google Scholar] [CrossRef]
- Kordt, M.; Trautmann, I.; Schlie, C.; Lindner, T.; Stenzel, J.; Schildt, A.; Boeckmann, L.; Bekeschus, S.; Kurth, J.; Krause, B.J.; et al. Multimodal imaging techniques to evaluate the anticancer effect of cold atmospheric pressure plasma. Cancers (Basel) 2021, 13, 2483. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Schuster, M.; Unger, J.; Seebauer, C.; Metelmann, H.R.; Woedtke, T.V.; Weltmann, K.D.; Daeschlein, G. Hyperspectral imaging for in vivo monitoring of cold atmospheric plasma effects on microcirculation in treatment of head and neck cancer and wound healing. Clin. Plas. Med. 2017, 7, 52–57. [Google Scholar] [CrossRef]
- Darny, T.; Pouvesle, J.M.; Puech, V.; Douat, C.; Dozias, S.; Robert, E. Analysis of conductive target influence in plasma jet experiments through helium metastable and electric field measurements. Plasma Sources Sci. T. 2017, 26, 045008. [Google Scholar] [CrossRef]
- Stancampiano, A.; Chung, T.H.; Dozias, S.; Pouvesle, J.M.; Mir, L.M.; Robert, E. Mimicking of human body electrical characteristic for easier translation of plasma biomedical studies to clinical applications. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 335–342. [Google Scholar] [CrossRef]
- Freund, E.; Bekeschus, S. Gas plasma-oxidized liquids for cancer treatment: Pre-clinical relevance, immuno-oncology, and clinical obstacles. IEEE Trans. Radiat. Plasma Med. Sci. 2020, accepted. [Google Scholar] [CrossRef]
- Clemen, R.; Freund, E.; Mrochen, D.; Miebach, L.; Schmidt, A.; Rauch, B.H.; Lackmann, J.W.; Martens, U.; Wende, K.; Lalk, M.; et al. Gas plasma technology augments ovalbumin immunogenicity and ot-ii t cell activation conferring tumor protection in mice. Adv. Sci. (Weinh) 2021, 8, 2003395. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kinpen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.; Wende, K.; Masur, K.; Iseni, S.; Dunnbier, M.; Hammer, M.U.; Tresp, H.; Weltmann, K.D.; Reuter, S. Feed gas humidity: A vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J. Phys. D Appl. Phys. 2013, 46, 295401. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.D.; Wende, K. Basic research in plasma medicine–a throughput approach from liquids to cells. J. Vis. Exp. 2017, e56331. [Google Scholar] [CrossRef] [Green Version]
- Miebach, L.; Freund, E.; Horn, S.; Niessner, F.; Sagwal, S.K.; von Woedtke, T.; Emmert, S.; Weltmann, K.D.; Clemen, R.; Schmidt, A.; et al. Tumor cytotoxicity and immunogenicity of a novel v-jet neon plasma source compared to the kinpen. Sci. Rep. 2021, 11, 136. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kinpen–a powerful tool for wound healing. Clin. Plas. Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Fox, M.H. A model for the computer analysis of synchronous DNA distributions obtained by flow cytometry. Cytometry 1980, 1, 71–77. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadati, F.; Moritz, J.; Berner, J.; Freund, E.; Miebach, L.; Helfrich, I.; Stoffels, I.; Emmert, S.; Bekeschus, S. Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet. Int. J. Mol. Sci. 2021, 22, 11446. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222111446
Saadati F, Moritz J, Berner J, Freund E, Miebach L, Helfrich I, Stoffels I, Emmert S, Bekeschus S. Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet. International Journal of Molecular Sciences. 2021; 22(21):11446. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222111446
Chicago/Turabian StyleSaadati, Fariba, Juliane Moritz, Julia Berner, Eric Freund, Lea Miebach, Iris Helfrich, Ingo Stoffels, Steffen Emmert, and Sander Bekeschus. 2021. "Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet" International Journal of Molecular Sciences 22, no. 21: 11446. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222111446





