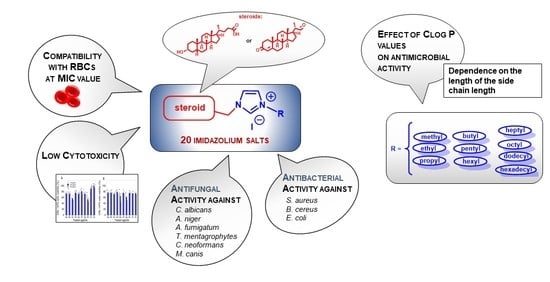
Steroid-Functionalized Imidazolium Salts with an Extended Spectrum of Antifungal and Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Imidazolium Salts
2.2. Antimicrobial Studies
2.3. Effect of ClogP Values on Antimicrobial Activity
2.4. Evaluation of the Hemolytic Activity of the Tested Compounds
2.5. Cytotoxicity Studies
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure for the Synthesis of Imidazolium Salts
3.2.1. N-(3α-Hydroxy-5β-cholan-24-yl)-N′-propylimidazolium Iodide (3c)
3.2.2. N-Butyl-N′-(3α-hydroxy-5β-cholan-24-yl)imidazolium Iodide (3d)
3.2.3. N-(3α-Hydroxy-5β-cholan-24-yl)-N′-pentylimidazolium Iodide (3e)
3.2.4. N-Hexyl-N′-(3α-hydroxy-5β-cholan-24-yl)imidazolium Iodide (3f)
3.2.5. N-Heptyl-N′-(3α-hydroxy-5β-cholan-24-yl)imidazolium Iodide (3g)
3.2.6. N-(3α-Hydroxy-5β-cholan-24-yl)-N′-octylimidazolium Iodide (3h)
3.2.7. N-Dodecyl-N′-(3α-hydroxy-5β-cholan-24-yl)imidazolium Iodide (3i)
3.2.8. N-Hexadecyl-N′-(3α-hydroxy-5β-cholan-24-yl)imidazolium Iodide (3j)
3.2.9. N-(3-Oxo-23,24-dinorchol-4-en-22-yl)-N′-propylimidazolium Iodide (4c)
3.2.10. N-Butyl-N′-(3-oxo-23,24-dinorchol-4-en-22-yl)imidazolium Iodide (4d)
3.2.11. N-(3-Oxo-23,24-dinorchol-4-en-22-yl)-N′-pentylimidazolium Iodide (4e)
3.2.12. N-Hexyl-N′-(3-oxo-23,24-dinorchol-4-en-22-yl)imidazolium Iodide (4f)
3.2.13. N-Heptyl-N′-(3-oxo-23,24-dinorchol-4-en-22-yl)imidazolium Iodide (4g)
3.2.14. N-Octyl-N′-(3-oxo-23,24-dinorchol-4-en-22-yl)imidazolium Iodide (4h)
3.2.15. N-Dodecyl-N′-(3-oxo-23,24-dinorchol-4-en-22-yl)imidazolium Iodide (4i)
3.2.16. N-Hexadecyl-N′-(3-oxo-23,24-dinorchol-4-en-22-yl)imidazolium Iodide (4j)
3.3. Antimicrobial Activity
3.4. Hemocompatibility Studies
3.5. Cytotoxicity Studies
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wall, G.; Lopez-Ribot, J.L. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef]
- Lorenz, M.C. Host-Microbe Interactions: Fungi. Curr. Opin. Microbiol. 2010, 13, 389–391. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z. The Modification of Natural Products for Medical Use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krcmery, V.; Kalavsky, E. Antifungal Drug Discovery, Six New Molecules Patented after 10 Years of Feast: Why Do We Need New Patented Drugs Apart from New Strategies? Recent Pat. Antiinfect. Drug Discov. 2007, 2, 182–187. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Deshpande, L.M.; Davis, A.P.; Rhomberg, P.R.; Pfaller, M.A. Monitoring Antifungal Resistance in a Global Collection of Invasive Yeasts and Molds: Application of CLSI Epidemiological Cutoff Values and Whole-Genome Sequencing Analysis for Detection of Azole Resistance in Candida Albicans. Antimicrob. Agents Chemother. 2017, 61, e00906-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, M.M.; Teixeira-Santos, R.; Silva, A.P.; Cruz, L.; Ricardo, E.; Pina-Vaz, C.; Rodrigues, A.G. The Effect of Antibacterial and Non-Antibacterial Compounds Alone or Associated with Antifugals upon Fungi. Front. Microbiol. 2015, 6, 669–673. [Google Scholar] [CrossRef]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-Bacterial Interactions in Health and Disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, E.F.; Kucharíková, S.; Van Dijck, P.; Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Clinical Implications of Oral Candidiasis: Host Tissue Damage and Disseminated Bacterial Disease. Infect. Immun. 2015, 83, 604–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, M.F.; Pereira, L.; Jenull, S.; Kuchler, K.; Lion, T. Klebsiella Pneumoniae Prevents Spore Germination and Hyphal Development of Aspergillus Species. Sci. Rep. 2019, 9, 218–233. [Google Scholar] [CrossRef] [Green Version]
- Reno, J.; Doshi, S.; Tunali, A.K.; Stein, B.; Farley, M.M.; Ray, S.M.; Jacob, J.T. Epidemiology of Methicillin-Resistant Staphylococcus Aureus Bloodstream Coinfection Among Adults With Candidemia in Atlanta, GA, 2008–2012. Infect. Control. Hosp. Epidemiol. 2015, 36, 1298–1304. [Google Scholar] [CrossRef]
- Stergiopoulou, T.; Meletiadis, J.; Sein, T.; Papaioannidou, P.; Tsiouris, I.; Roilides, E.; Walsh, T.J. Comparative Pharmacodynamic Interaction Analysis between Ciprofloxacin, Moxifloxacin and Levofloxacin and Antifungal Agents against Candida Albicans and Aspergillus Fumigatus. J. Antimicrob. Chemother. 2009, 63, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida Albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2017, 7, 2173–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrekker, H.S.; Donato, R.K.; Fuentefria, A.M.; Bergamo, V.; Oliveira, L.F.; Machado, M.M. Imidazolium Salts as Antifungal Agents: Activity against Emerging Yeast Pathogens, without Human Leukocyte Toxicity. Med. Chem. Commun. 2013, 4, 1457–1460. [Google Scholar] [CrossRef]
- Ribas, A.D.; Del Ponte, E.M.; Dalbem, A.M.; Dalla-Lana, D.; Bündchen, C.; Donato, R.K.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium Salts with Antifungal Potential for the Control of Head Blight of Wheat Caused by Fusarium Graminearum. J. Appl. Microbiol. 2016, 121, 445–452. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Imidazolium Salts and Their Polymeric Materials for Biological Applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef]
- Bando, T.; Hiroshi, S. Synthesis and Biological Properties of Sequence-Specific DNA-Alkylating Pyrrole−Imidazole Polyamides. Acc. Chem. Res. 2006, 39, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Bredas, J.L.; Poskin, M.P.; Delhalle, J.; Andre, J.M.; Chojnacki, H. Electronic Structure of Hydrogen-Bonded Imidazole Chains. Influence of the Proton Position. J. Phys. Chem. 1984, 88, 5882–5887. [Google Scholar] [CrossRef]
- Ganapathi, P.; Ganesan, K.; Vijaykanth, N.; Arunagirinathan, N. Anti-Bacterial Screening of Water Soluble Carbonyl Diimidazolium Salts and Its Derivatives. J. Mol. Liq. 2016, 219, 180–185. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and Imidazolium-Containing Polymers for Biology and Material Science Applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef] [Green Version]
- Ghanta, K.P.; Pal, T.; Mondal, S.; Bandyopadhyay, S. Microscopic Understanding of the Effect of Ionic Liquid on Protein from Molecular Simulation Studies. J. Phys. Chem. B 2020, 124, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Manogaran, P.; Gadepalli Narasimha, K.K.; Kalpattu Kuppusami, B.; Mariyappan, P.; Gopalakrishnan, A.; Venkatraman, G. Synthesis and Anticancer Activity of Novel Curcumin–Quinolone Hybrids. Bioorg. Med. Chem. Lett. 2015, 25, 3601–3605. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Y.; Xu, W.; Wang, Y.; Guo, X. Recent Progress in the Assembly Behavior of Imidazolium-Based Ionic Liquid Surfactants. J. Mol. Liq. 2020, 319, 114354–114374. [Google Scholar] [CrossRef]
- Padvi, S.A.; Dalal, D.S. Task-Specific Ionic Liquids as a Green Catalysts and Solvents for Organic Synthesis. Curr. Green Chem. 2020, 7, 105–119. [Google Scholar] [CrossRef]
- De Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, Properties, and Organometallic Chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef] [PubMed]
- Chábera, P.; Lindh, L.; Rosemann, N.W.; Prakash, O.; Uhlig, J.; Yartsev, A.; Wärnmark, K.; Sundström, V.; Persson, P. Photofunctionality of Iron(III) N-Heterocyclic Carbenes and Related D5 Transition Metal Complexes. Coord. Chem. Rev. 2021, 426, 213517–213539. [Google Scholar] [CrossRef]
- Reshi, N.U.D.; Bera, J.K. Recent Advances in Annellated NHCs and Their Metal Complexes. Coord. Chem. Rev. 2020, 422, 213334–213403. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Jin, Z.; Chi, Y.R. N-Heterocyclic Carbene Organocatalysis: Activation Modes and Typical Reactive Intermediates. Chin. J. Chem. 2020, 38, 1167–1202. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Kafshdarzadeh, K.; Amiri, Z. Recent Advances in Stetter Reaction and Related Chemistry: An Update. Asian J. Org. Chem. 2020, 9, 1999–2034. [Google Scholar] [CrossRef]
- Liu, J.; Xing, X.-N.; Huang, J.-H.; Lu, L.-Q.; Xiao, W.-J. Light Opens a New Window for N-Heterocyclic Carbene Catalysis. Chem. Sci. 2020, 11, 10605–10613. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal Applications of Imidazolium Carbene−Metal Complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, A.; Cisnetti, F. Advances in Metal–Carbene Complexes as Potent Anti-Cancer Agents. Metallomics 2012, 4, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicka, A.; Malinowska, M.; Hauschild, T.; Pieczul, K.; Morzycki, J.W. Synthesis and Antimicrobial Properties of Steroid-Based Imidazolium Salts. J. Steroid Biochem. Mol. Biol. 2019, 189, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tenneson, M.E.; Bilton, R.F.; Mason, A.N. The Degradation of Lithocholic Acid by Pseudomonas Spp NCIB 10590. FEBS Lett. 1978, 91, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Hryniewicka, A.; Niemirowicz-Laskowska, K.; Wielgat, P.; Car, H.; Hauschild, T.; Morzycki, J.W. Dehydroepiandrosterone Derived Imidazolium Salts and Their Antimicrobial Efficacy. Bioorg. Chem. 2021, 108, 104550–104561. [Google Scholar] [CrossRef]
- Salas, P.F.; Herrmann, C.; Cawthray, J.F.; Nimphius, C.; Kenkel, A.; Chen, J.; de Kock, C.; Smith, P.J.; Patrick, B.O.; Adam, M.J.; et al. Structural Characteristics of Chloroquine-Bridged Ferrocenophane Analogues of Ferroquine May Obviate Malaria Drug-Resistance Mechanisms. J. Med. Chem. 2013, 56, 1596–1613. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Bheemanaboina, R.R.Y.; Gao, W.-W.; Kang, J.; Cai, G.-X.; Zhou, C.-H. Discovery of Benzimidazole–Quinolone Hybrids as New Cleaving Agents toward Drug-Resistant Pseudomonas Aeruginosa DNA. ChemMedChem 2018, 13, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Yusof, M.Y.M.; Hassan, H.; Sekaran, S.D. In Vitro Properties of Designed Antimicrobial Peptides That Exhibit Potent Antipneumococcal Activity and Produces Synergism in Combination with Penicillin. Sci. Rep. 2015, 5, 9761–9769. [Google Scholar] [CrossRef] [Green Version]
- Pick, N.; Cameron, S.; Arad, D.; Av-Gay, Y. Screening of Compounds Toxicity against Human Monocytic Cell Line-THP-1 by Flow Cytometry. Biol. Proced. Online 2004, 6, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhou, J.; Xu, Y. Study of the in Vitro Cytotoxicity Testing of Medical Devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; CLSI Document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Rodrigo, C.; Weeratunga, P.; Fernando, S.D.; Rajapakse, S. Amphotericin B for Treatment of Visceral Leishmaniasis: Systematic Review and Meta-Analysis of Prospective Comparative Clinical Studies Including Dose-Ranging Studies. Clin. Microbiol. Infect. 2018, 24, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Salt | Reaction Temperature | Reaction Time | Yield | Salt | Reaction Temperature | Reaction Time | Yield | Number of Carbon Atoms in the Alkyl Substituent |
|---|---|---|---|---|---|---|---|---|
| 3a a | r.t. | 24 h | 83% | 4a a | r.t. | 20 h | 100% | 1 |
| 3b a | r.t. | 24 h | 64% | 4b a | r.t. | 20 h | 99% | 2 |
| 3c | 80 °C | 2 h | 74% | 4c | 80 °C | 0.25 h | 99% | 3 |
| 3d | 80 °C | 2 h | 71% | 4d | 80 °C | 1 h | 85% | 4 |
| 3e | 80 °C | 2 h | 72% | 4e | 80 °C | 1 h | 79% | 5 |
| 3f | 80 °C | 2 h | 72% | 4f | 80 °C | 1.5 h | 100% | 6 |
| 3g | 80 °C | 2 h | 70% | 4g | 80 °C | 3 h | 73% | 7 |
| 3h | 80 °C | 2 h | 74% | 4h | 100 °C | 2 h | 93% | 8 |
| 3i | 80 °C | 2 h | 64% | 4i | 100 °C | 1.5 h | 81% | 12 |
| 3j | 80 °C | 2 h | 55% | 4j | 100 °C | 2 h | 67% | 16 |
| MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Salt | S. aureus | B. cereus | E. coli | Salt | S. aureus | B. cereus | E. coli |
| 3a | 4 a | 32 a | 16 a | 4a | 32 a | 64 a | 16 a |
| 3b | 2 a | 16 a | 16 a | 4b | 16 a | 32 a | 8 a |
| 3c | 1 | 8 | 16 | 4c | 16 | 16 | 8 |
| 3d | 0.5 | 4 | 16 | 4d | 8 | 16 | 8 |
| 3e | 1 a | 4 a | 16 a | 4e | 4 a | 4 a | 4 a |
| 3f | 0.5 a | 2 a | 16 a | 4f | 2 a | 2 a | 4 a |
| 3g | 0.06 | 2 | 16 | 4g | 0.5 | 0.5 | 4 |
| 3h | 0.06 | 2 | 64 | 4h | 0.25 | 0.5 | 4 |
| 3i | 2 | 4 | 512 | 4i | 0.5 | 1 | 512 |
| 3j | 16 | 64 | 512 | 4j | 4 | 8 | 512 |
| Ampicillin | 0.25–1 | 0.25–0.5 | 2–8 | ||||
| MIC (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Salt | C. albicans | A. niger | A. fumigatus | T. mentagrophytes | C. neoformans | M. canis |
| 3a | 0.25 a | 4 | 4 | 0.25 | 1 | 0.25 |
| 3b | 0.5 a | 1 | 1 | 0.5 | 1 | 0.25 |
| 3c | 0.25 | 16 | 8 | 2 | 2 | 0.25 |
| 3d | 0.5 | 16 | 8 | 2 | 4 | 0.25 |
| 3e | 0.5 a | 2 | 8 | 0.5 | 2 | 0.25 |
| 3f | 0.5 a | 4 | 8 | 0.5 | 4 | 0.25 |
| 3g | 0.5 | 8 | 16 | 1 | 8 | 0.5 |
| 3h | 1 | 8 | 16 | 2 | 8 | 1 |
| 3i | 8 | 64 | 16 | 4 | 32 | 2 |
| 3j | 32 | 64 | 64 | 32 | 64 | 16 |
| 4a | 2 a | 8 | 2 | 2 | 4 | 0.5 |
| 4b | 1 a | 2 | 4 | 2 | 2 | 0.25 |
| 4c | 0.06 | 16 | 8 | 2 | 2 | 0.5 |
| 4d | 0.06 | 8 | 8 | 1 | 2 | 0.25 |
| 4e | 2 a | 8 | 8 | 2 | 4 | 0.5 |
| 4f | 1 a | 2 | 8 | 1 | 2 | 0.25 |
| 4g | 0.5 | 8 | 16 | 1 | 4 | 0.25 |
| 4h | 0.5 | 4 | 16 | 1 | 4 | 0.25 |
| 4i | 0.5 | 4 | 4 | 1 | 4 | 0.25 |
| 4j | 8 | 16 | 32 | 4 | 32 | 2 |
| Fluconazole | 0.5 | 16 | 64 | 64 | 4 | 32 |
| Amphotericin B | 0.5 | 2 | 2 | 1 | 0.5 | 1 |
| Voriconazole | 0.25 | 0.5 | 0.25 | 0.5 | 0.125 | 1 |
| Salt | ClogP | Salt | ClogP |
|---|---|---|---|
| 3a | 3.467 | 4a | 1.863 |
| 3b | 3.996 | 4b | 2.392 |
| 3c | 4.525 | 4c | 2.921 |
| 3d | 5.054 | 4d | 3.450 |
| 3e | 5.583 | 4e | 3.979 |
| 3f | 6.112 | 4f | 4.508 |
| 3g | 7.170 | 4g | 5.566 |
| 3h | 8.228 | 4h | 6.624 |
| 3i | 9.286 | 4i | 7.682 |
| 3j | 11.402 | 4j | 9.798 |
| HC50 (µg/mL) | |||
|---|---|---|---|
| Salt | RBC | Salt | RBC |
| 3a | 265.1 | 4a | nd |
| 3b | 44.6 | 4b | nd |
| 3c | 54.7 | 4c | nd |
| 3d | 53.7 | 4d | nd |
| 3e | 39.9 | 4e | 401.4 |
| 3f | 42.3 | 4f | 43.9 |
| 3g | 12.2 | 4g | 57.6 |
| 3h | 541.6 | 4h | 33.8 |
| 3i | nd | 4i | 54.5 |
| 3j | nd | 4j | 367.3 |
| IC50 (µg/mL) | Viability at MIC Value (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salt | CRL-1475 | THP-1 | Salt | CRL-1475 | THP-1 | Salt | CRL-1475 | THP-1 | Salt | CRL-1475 | THP-1 |
| 3a | 107.4 | nd | 4a | 469.0 | 562.9 | 3a | 100.6 ± 2.5 | 111.88 ± 22.5 | 4a | 100.6 ± 3.1 | 112.69 ± 2.59 |
| 3b | 113.9 | 205.3 | 4b | 524.2 | nd | 3b | 102.6 ± 1.5 | 122.4 ± 5.53 | 4b | 100.2 ± 2.3 | 130.51 ± 4.75 |
| 3c | 36.5 | 452.9 | 4c | 562.4 | 352.1 | 3c | 98.0 ± 4.3 | 95.11 ± 7.09 | 4c | 98.2 ± 1.4 | 105.38 ± 6.42 |
| 3d | 99.2 | 976.8 | 4d | 446.3 | 96.33 | 3d | 101.7 ± 2.3 | 99.82 ± 16.78 | 4d | 101.3 ± 2.0 | 109.39 ± 4.65 |
| 3e | 100.8 | 77.43 | 4e | 42.9 | 295.8 | 3e | 99.9 ± 2.6 | 123.59 ± 16.79 | 4e | 100.9 ± 1.5 | 71,49 ± 1.49 |
| 3f | 149.6 | nd | 4f | 717.7 | 313.2 | 3f | 102.3 ± 5.4 | 85.24 ± 16.19 | 4f | 99.8 ± 0.6 | 96.07 ± 13.97 |
| 3g | 134.4 | nd | 4g | 51.5 | 238.4 | 3g | 99.7 ± 3.4 | 85.24 ± 21.53 | 4g | 96.2 ± 1.9 | 87.07 ± 12.86 |
| 3h | 53.6 | 752.2 | 4h | 598.8 | 336.6 | 3h | 102.7 ± 2.9 | 119.57 ± 15.02 | 4h | 96.9 ± 2.2 | 106.68 ± 5.17 |
| 3i | 631.0 | nd | 4i | 457.7 | 713.9 | 3i | 80.5 ± 0.8 | 120.62 ± 17.80 | 4i | 100.2 ± 0.7 | 101.60 ± 13.22 |
| 3j | 841.1 | 451.4 | 4j | 282.3 | 853.4 | 3j | 101.26 ± 2.7 | 128.68 ± 14.53 | 4j | 81.3 ± 2.4 | 88.10 ± 19.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowska, M.; Sawicka, D.; Niemirowicz-Laskowska, K.; Wielgat, P.; Car, H.; Hauschild, T.; Hryniewicka, A. Steroid-Functionalized Imidazolium Salts with an Extended Spectrum of Antifungal and Antibacterial Activity. Int. J. Mol. Sci. 2021, 22, 12180. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222212180
Malinowska M, Sawicka D, Niemirowicz-Laskowska K, Wielgat P, Car H, Hauschild T, Hryniewicka A. Steroid-Functionalized Imidazolium Salts with an Extended Spectrum of Antifungal and Antibacterial Activity. International Journal of Molecular Sciences. 2021; 22(22):12180. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222212180
Chicago/Turabian StyleMalinowska, Marta, Diana Sawicka, Katarzyna Niemirowicz-Laskowska, Przemysław Wielgat, Halina Car, Tomasz Hauschild, and Agnieszka Hryniewicka. 2021. "Steroid-Functionalized Imidazolium Salts with an Extended Spectrum of Antifungal and Antibacterial Activity" International Journal of Molecular Sciences 22, no. 22: 12180. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222212180