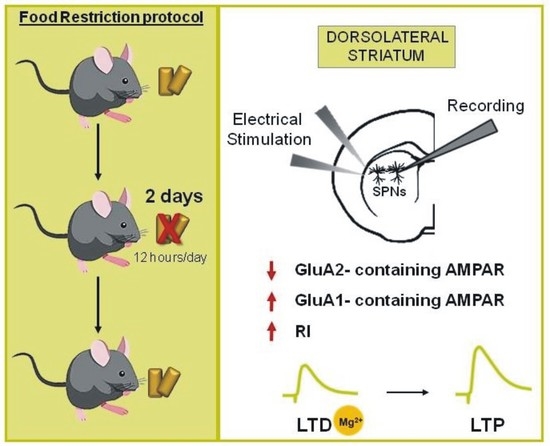
Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting
Abstract
:1. Introduction
2. Results
2.1. Acute Food Restriction Protocol Induced Long-term Changes in Spontaneous Glutamatergic Synaptic Currents in the Corticostriatal Synapses
2.2. SPNs of FR Mice Showed Increased Inwardly Rectifying AMPARs Currents and Unbalanced AMPA:NMDA Ratio
2.3. Enhanced GluA1-mediated Function in Striatal SPNs of FR Mice was associated with a Change in the Direction of Corticostriatal Synaptic Plasticity
2.4. Selective GluA1 Antagonism was Associated with the Reappearance of Corticostriatal LTD in FR Mice
2.5. Enhanced GluA1-AMPARs Function was Associated with Changes in LTP Maintenance in Striatal SPNs of FR Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Slices Preparation
4.3. Whole-cell Patch-clamp Recordings
4.4. Intracellular Recordings with Sharp Electrodes
4.5. Chemicals
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, L.T.; Mitchell, J.R. Benefits of short-term dietary restriction in mammals. Exp. Gerontol. 2013, 48, 1043–1048. [Google Scholar] [CrossRef] [Green Version]
- Trepanowski, J.F.; Bloomer, R.J. The impact of religious fasting on human health. Nutr. J. 2010, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Trepanowski, J.F.; Canale, R.E.; Marshall, K.E.; Kabir, M.M.; Bloomer, R.J. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benau, E.M.; Orloff, N.C.; Janke, E.A.; Serpell, L.; Timko, C.A. A systematic review of the effects of experimental fasting on cognition. Appetite 2014, 77, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Chechko, N.; Vocke, S.; Habel, U.; Toygar, T.; Kuckartz, L.; Berthold-Losleben, M.; Laoutidis, Z.G.; Orfanos, S.; Wassenberg, A.; Karges, W.; et al. Effects of overnight fasting on working memory-related brain network: An fMRI study. Hum. Brain Mapp. 2015, 36, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Solianik, R.; Sujeta, A.; Terentjevienė, A.; Skurvydas, A. Effect of 48 h Fasting on Autonomic Function, Brain Activity, Cognition, and Mood in Amateur Weight Lifters. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Deng, J.; Chen, S.; Que, J.; Sun, Y.; Wang, Z.; Guo, X.; Han, Y.; Zhou, Y.; Zhang, X.; et al. Fasting enhances extinction retention and prevents the return of fear in humans. Transl. Psychiatry 2018, 8, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.C.L. Fasting as a Therapy in Neurological Disease. Nutrients 2019, 11, 2501. [Google Scholar] [CrossRef] [Green Version]
- Fontán-Lozano, A.; Sáez-Cassanelli, J.L.; Inda, M.C.; de los Santos-Arteaga, M.; Sierra-Domínguez, S.A.; López-Lluch, G.; Delgado-García, J.M.; Carrión, A.M. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J. Neurosci 2007, 27, 10185–10195. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, Z.; Zuo, Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS ONE 2013, 8, e66069. [Google Scholar] [CrossRef] [Green Version]
- Babits, R.; Szőke, B.; Sótonyi, P.; Rácz, B. Food restriction modifies ultrastructure of hippocampal synapses. Hippocampus 2016, 26, 437–444. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Catarino, T.; Santos, S.D.; Benoist, M.; van Leeuwen, J.F.; Esteban, J.A.; Carvalho, A.L. Ghrelin triggers the synaptic incorporation of AMPA receptors in the hippocampus. Proc. Natl. Acad. Sci. USA 2014, 111, E149–E158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Carcea, I.; Schiavo, J.K.; Jones, K.T.; Rabinowitsch, A.; Kolaric, R.; Cabeza de Vaca, S.; Froemke, R.C.; Carr, K.D. Food restriction induces synaptic incorporation of calcium-permeable AMPA receptors in nucleus accumbens. Eur. J. Neurosci. 2017, 45, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.D.; Tsimberg, Y.; Berman, Y.; Yamamoto, N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 2003, 119, 1157–1167. [Google Scholar] [CrossRef]
- Haberny, S.L.; Carr, K.D. Comparison of basal and D-1 dopamine receptor agonist-stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Brain Res. Mol. Brain Res. 2005, 141, 121–127. [Google Scholar] [CrossRef]
- Shi, S.; Hayashi, Y.; Esteban, J.A.; Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 2001, 105, 331–343. [Google Scholar] [CrossRef]
- Esteban, J.A.; Shi, S.H.; Wilson, C.; Nuriya, M.; Huganir, R.L.; Malinow, R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003, 6, 136–143. [Google Scholar] [CrossRef]
- Man, H.Y.; Sekine-Aizawa, Y.; Huganir, R.L. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. USA 2007, 104, 3579–3584. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Song, L.; Cummings, L.W.; Goldman, J.; Huganir, R.L.; Lee, H.K. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 20033–20038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clem, R.L.; Huganir, R.L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 2010, 330, 1108–1112. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, J.L.; Gorski, J.A.; Dell’Acqua, M.L. NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca(2)(+)-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron 2016, 89, 1000–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.L.; Aarts, E.; Dang, L.C.; Greer, S.M.; Jagust, W.J.; D’Esposito, M. Dorsal striatal dopamine, food preference and health perception in humans. PLoS ONE 2014, 9, e96319. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: Beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, 15037–15042. [Google Scholar] [CrossRef] [Green Version]
- Carr, K.D.; Cabeza de Vaca, S.; Sun, Y.; Chau, L.S. Reward-potentiating effects of D-1 dopamine receptor agonist and AMPAR GluR1 antagonist in nucleus accumbens shell and their modulation by food restriction. Psychopharmacology 2009, 202, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Jeun, S.H.; Cho, H.S.; Kim, K.J.; Li, Q.Z.; Sung, K.W. Electrophysiological Characterization of AMPA and NMDA Receptors in Rat Dorsal Striatum. Korean J. Physiol. Pharmacol. 2009, 13, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greger, I.H.; Khatri, L.; Ziff, E.B. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 2002, 34, 759–772. [Google Scholar] [CrossRef] [Green Version]
- Nansen, E.A.; Jokel, E.S.; Lobo, M.K.; Micevych, P.E.; Ariano, M.A.; Levine, M.S. Striatal ionotropic glutamate receptor ontogeny in the rat. Dev. Neurosci. 2000, 22, 329–340. [Google Scholar] [CrossRef]
- Boulter, J.; Hollmann, M.; O’Shea-Greenfield, A.; Hartley, M.; Deneris, E.; Maron, C.; Heinemann, S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science 1990, 249, 1033–1037. [Google Scholar] [CrossRef]
- Verdoorn, T.A.; Burnashev, N.; Monyer, H.; Seeburg, P.H.; Sakmann, B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science 1991, 252, 1715–1718. [Google Scholar] [CrossRef]
- Geiger, J.R.; Melcher, T.; Koh, D.S.; Sakmann, B.; Seeburg, P.H.; Jonas, P.; Monyer, H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 1995, 15, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.J.; Brenowitz, S.; Trussell, L.O. The mechanism of action of aniracetam at synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors: Indirect and direct effects on desensitization. Mol. Pharmacol. 2003, 64, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, P.; Maj, R.; Pisani, A.; Mercuri, N.B.; Bernardi, G. Long-term synaptic depression in the striatum: Physiological and pharmacological characterization. J. Neurosci. 1992, 12, 4224–4233. [Google Scholar] [CrossRef]
- Martin, L.J.; Furuta, A.; Blackstone, C.D. AMPA receptor protein in developing rat brain: Glutamate receptor-1 expression and localization change at regional, cellular, and subcellular levels with maturation. Neuroscience 1998, 83, 917–928. [Google Scholar] [CrossRef]
- Kumar, S.S.; Bacci, A.; Kharazia, V.; Huguenard, J.R. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J. Neurosci. 2002, 22, 3005–3015. [Google Scholar] [CrossRef] [Green Version]
- Bellone, C.; Mameli, M.; Luscher, C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat. Neurosci. 2011, 14, 1439–1446. [Google Scholar] [CrossRef]
- Eybalin, M.; Caicedo, A.; Renard, N.; Ruel, J.; Puel, J.L. Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur. J. Neurosci. 2004, 20, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.T.; Pelkey, K.A.; Topolnik, L.; Petralia, R.S.; Takamiya, K.; Xia, J.; Huganir, R.L.; Lacaille, J.C.; McBain, C.J. Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J. Neurosci. 2007, 27, 11651–11662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derkach, V.; Barria, A.; Soderling, T.R. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 3269–3274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, J.T.; Ashby, M.C.; McBain, C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 2007, 54, 859–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Agopyan, N.; Miu, P.; Xiong, Z.; Henderson, J.; Gerlai, R.; Taverna, F.A.; Velumian, A.; MacDonald, J.; Carlen, P.; et al. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 1996, 17, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Fujiyama, F.; Kuramoto, E.; Okamoto, K.; Hioki, H.; Furuta, T.; Zhou, L.; Nomura, S.; Kaneko, T. Presynaptic localization of an AMPA-type glutamate receptor in corticostriatal and thalamostriatal axon terminals. Eur. J. Neurosci. 2004, 20, 3322–3330. [Google Scholar] [CrossRef]
- Patel, D.R.; Young, A.M.; Croucher, M.J. Presynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor-mediated stimulation of glutamate and GABA release in the rat striatum in vivo: A dual-label microdialysis study. Neuroscience 2001, 102, 101–111. [Google Scholar] [CrossRef]
- Dohovics, R.; Janaky, R.; Varga, V.; Hermann, A.; Saransaari, P.; Oja, S.S. Regulation of glutamatergic neurotransmission in the striatum by presynaptic adenylyl cyclase-dependent processes. Neurochem. Int. 2003, 42, 1–7. [Google Scholar] [CrossRef]
- Rouse, S.T.; Marino, M.J.; Bradley, S.R.; Awad, H.; Wittmann, M.; Conn, P.J. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: Implications for treatment of Parkinson’s disease and related disorders. Pharmacol. Ther. 2000, 88, 427–435. [Google Scholar] [CrossRef]
- Laricchiuta, D.; Rossi, S.; Musella, A.; De Chiara, V.; Cutuli, D.; Centonze, D.; Petrosini, L. Differences in spontaneously avoiding or approaching mice reflect differences in CB1-mediated signaling of dorsal striatal transmission. PLoS ONE 2012, 7, e33260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagetta, V.; Picconi, B.; Marinucci, S.; Sgobio, C.; Pendolino, V.; Ghiglieri, V.; Fusco, F.R.; Giampà, C.; Calabresi, P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: Implications for Parkinson’s disease. J. Neurosci. 2011, 31, 12513–12522. [Google Scholar] [CrossRef] [Green Version]
- Bagetta, V.; Sgobio, C.; Pendolino, V.; Del Papa, G.; Tozzi, A.; Ghiglieri, V.; Giampà, C.; Zianni, E.; Gardoni, F.; Calabresi, P.; et al. Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson’s disease. J. Neurosci. 2012, 32, 17921–17931. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, P.; Pisani, A.; Mercuri, N.B.; Bernardi, G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur. J. Neurosci. 1992, 4, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Fino, E.; Vandecasteele, M.; Perez, S.; Saudou, F.; Venance, L. Region-specific and state-dependent action of striatal GABAergic interneurons. Nat. Commun. 2018, 9, 3339. [Google Scholar] [CrossRef] [Green Version]
- Hawes, S.L.; Salinas, A.G.; Lovinger, D.M.; Blackwell, K.T. Long-term plasticity of corticostriatal synapses is modulated by pathway-specific co-release of opioids through kappa-opioid receptors. J. Physiol. 2017, 595, 5637–5652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlak, V.; Kerr, J.N. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 2008, 28, 2435–2446. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanelli, F.; Laricchiuta, D.; Natale, G.; Marino, G.; Calabrese, V.; Picconi, B.; Petrosini, L.; Calabresi, P.; Ghiglieri, V. Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting. Int. J. Mol. Sci. 2021, 22, 1916. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041916
Campanelli F, Laricchiuta D, Natale G, Marino G, Calabrese V, Picconi B, Petrosini L, Calabresi P, Ghiglieri V. Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting. International Journal of Molecular Sciences. 2021; 22(4):1916. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041916
Chicago/Turabian StyleCampanelli, Federica, Daniela Laricchiuta, Giuseppina Natale, Gioia Marino, Valeria Calabrese, Barbara Picconi, Laura Petrosini, Paolo Calabresi, and Veronica Ghiglieri. 2021. "Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting" International Journal of Molecular Sciences 22, no. 4: 1916. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041916