Alternative to Poly(2-isopropyl-2-oxazoline) with a Reduced Ability to Crystallize and Physiological LCST
Abstract
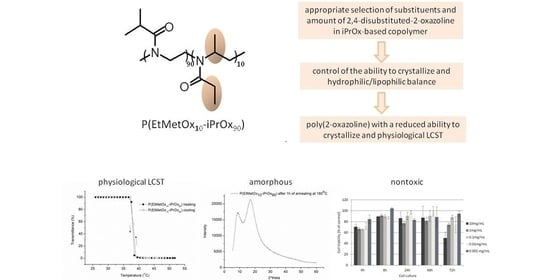
:1. Introduction
2. Results and Discussion
2.1. Properties in the Aqueous Solution
2.2. Properties in the Solid-State
2.3. The Role of the R2 Methyl Substituent
2.4. Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Copolymers
3.3. Measurements
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of poly(NIPAM) over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, X.; Wu, C. Comparison of the coil-to-globule and the globule-to-coil transitions of a single poly(N-isopropylacrylamide) homopolymer chain in water. Macromolecules 1998, 31, 2972–2976. [Google Scholar] [CrossRef]
- Huber, S.; Jordan, R. Modulation of the lower critical solution temperature of 2-Alkyl-2-oxazoline copolymers. Colloid Polym. Sci. 2008, 286, 395–402. [Google Scholar] [CrossRef]
- Diab, C.; Akiyama, Y.; Kataoka, K.; Winnik, F.M. Microcalorimetric Study of the Temperature-Induced Phase Separation in Aqueous Solutions of Poly(2-isopropyl-2-oxazolines). Macromolecules 2004, 37, 2556–2562. [Google Scholar] [CrossRef]
- Aoi, K.; Okada, M. Polymerization of Oxazolines. Prog. Polym. Sci. 1996, 21, 151–208. [Google Scholar] [CrossRef]
- Park, J.S.; Akiyama, Y.; Winnik, F.M.; Kataoka, K. Versatile synthesis of end-functionalized thermosensitive poly(2-isopropyl-2-oxazolines). Macromolecules 2004, 37, 6786–6792. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Sahay, G.; Schulz, A.; Alakhova, D.; Bronich, T.K.; Jordan, R.; Kabanov, A.V. Structure-property relationship in cytotoxicity and cell uptake of poly(2-oxazoline) amphiphiles. J. Control. Release 2011, 153, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Toncheva-Moncheva, N.; Veleva-Kostadinova, E.; Tsvetanov, C.; Momekova, D.; Rangelov, S. Preparation and properties of positively charged mesoglobules based on poly(2-isopropyl-2-oxazoline) and evaluation of their potential as carriers of polynucleotides. Polymer (Guildf.) 2017, 111, 156–167. [Google Scholar] [CrossRef]
- Oleszko, N.; Wałach, W.; Utrata-Wesołek, A.; Kowalczuk, A.; Trzebicka, B.; Klama-Baryła, A.; Hoff-Lenczewska, D.; Kawecki, M.; Lesiak, M.; Sieroń, A.L.; et al. Controlling the crystallinity of thermoresponsive poly(2-oxazoline)-based nanolayers to cell adhesion and detachment. Biomacromolecules 2015, 16, 2805–2813. [Google Scholar] [CrossRef]
- Dworak, A.; Utrata-Wesołek, A.; Oleszko, N.; Wałach, W.; Trzebicka, B.; Anioł, J.; Sieroń, A.L.; Klama-Baryła, A.; Kawecki, M. Poly(2-substituted-2-oxazoline) surfaces for dermal fibroblasts adhesion and detachment. J. Mater. Sci. Mater. Med. 2014, 25, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Uyama, H.; Kobayashi, S. A Novel Thermo-Sensitive Polymer. Poly(2- iso -propyl-2-oxazoline). Chem. Lett. 1992, 21, 1643–1646. [Google Scholar] [CrossRef]
- Meyer, M.; Antonietti, M.; Schlaad, H. Unexpected thermal characteristics of aqueous solutions of poly(2-isopropyl-2-oxazoline). Soft Matter 2007, 3, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Demirel, A.L.; Meyer, M.; Schlaad, H. Formation of polyamide nanofibers by directional crystallization in aqueous solution. Angew. Chem. Int. Ed. 2007, 46, 8622–8624. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Rogers, S.; Schubert, U.S.; Hoogenboom, R. Secondary structure formation of main-chain chiral poly(2-oxazoline)s in solution. Soft Matter 2010, 6, 994–1003. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Conformational changes in the heat-induced crystallization of poly(2-isopropyl-2-oxazoline) in the solid state. Phys. Chem. Chem. Phys. 2015, 17, 31084–31092. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Tsuchiizu, A.; Qiu, X.; Winnik, F.M. Dissecting the mechanism of the heat-induced phase separation and crystallization of poly(2-isopropyl-2-oxazoline) in water through vibrational spectroscopy and molecular orbital calculations. Macromolecules 2012, 45, 3531–3541. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. From globules to crystals: A spectral study of poly(2-isopropyl-2-oxazoline) crystallization in hot water. Phys. Chem. Chem. Phys. 2015, 17, 32232–32240. [Google Scholar] [CrossRef]
- Li, T.; Tang, H.; Wu, P. Molecular Evolution of Poly(2-isopropyl-2-oxazoline) Aqueous Solution during the Liquid-Liquid Phase Separation and Phase Transition Process. Langmuir 2015, 31, 6870–6878. [Google Scholar] [CrossRef] [PubMed]
- Furuncuoğlu Özaltın, T.; Aviyente, V.; Atılgan, C.; Demirel, L. Multiscale modeling of poly(2-isopropyl-2-oxazoline) chains in aqueous solution. Eur. Polym. J. 2017, 88, 594–604. [Google Scholar] [CrossRef]
- Morimoto, N.; Obeid, R.; Yamane, S.; Winnik, F.M.; Akiyoshi, K. Composite nanomaterials by self-assembly and controlled crystallization of poly(2-isopropyl-2-oxazoline)-grafted polysaccharides. Soft Matter 2009, 5, 1597–1600. [Google Scholar] [CrossRef]
- Diehl, C.; Schlaad, H. Polyoxazoline-based crystalline microspheres for carbohydrate-protein recognition. Chem. Eur. J. 2009, 15, 11469–11472. [Google Scholar] [CrossRef] [PubMed]
- Oleszko-Torbus, N.; Wałach, W.; Utrata-Wesołek, A.; Dworak, A. Control of the Crystalline Properties of 2-Isopropyl-2-oxazoline Copolymers in Condensed State and in Solution Depending on the Composition. Macromolecules 2017, 50, 7636–7645. [Google Scholar] [CrossRef]
- Legros, C.; De Pauw-Gillet, M.C.; Tam, K.C.; Taton, D.; Lecommandoux, S. Crystallisation-driven self-assembly of poly(2-isopropyl-2-oxazoline)-block-poly(2-methyl-2-oxazoline) above the LCST. Soft Matter 2015, 11, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Oleszko, N.; Utrata-Wesołek, A.; Wałach, W.; Libera, M.; Hercog, A.; Szeluga, U.; Domański, M.; Trzebicka, B.; Dworak, A. Crystallization of Poly(2-isopropyl-2-oxazoline) in Organic Solutions. Macromolecules 2015, 48, 1852–1859. [Google Scholar] [CrossRef]
- Oleszko-Torbus, N.; Mendrek, B.; Kowalczuk, A.; Utrata-Wesołek, A.; Dworak, A.; Wałach, W. Selective partial hydrolysis of 2-isopropyl-2-oxazoline copolymers towards decreasing the ability to crystallize. Materials 2020, 13, 3403. [Google Scholar] [CrossRef]
- Yahiaoui, A.; Hachemaoui, A.; Belbachir, M. Synthesis of hydrosoluble polymers of oxazoline using Maghnite-H as catalyst. J. Appl. Polym. Sci. 2007, 104, 1792–1800. [Google Scholar] [CrossRef]
- Saegusa, T.; Kimura, Y.; Sawada, S.; Kobayashi, S. A New Route to Optically Active Linear Poly(propylenimine). Macromolecules 1974, 7, 958–959. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Huber, S.; Hytry, J.; Tong, J.; Kabanov, A.V.; Jordan, R. Chiral and water-soluble poly(2-oxazoline)s. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 732–738. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Hendrix, M.M.R.M.; Schubert, U.S.; Hoogenboom, R. Ordered chiral structures in the crystals of main-chain chiral poly(2-oxazoline)s. Macromolecules 2010, 43, 4654–4659. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Schubert, U.S.; Hoogenboom, R. Main-chain chiral copoly(2-oxazoline)s. Polym. Chem. 2011, 2, 203–208. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Hoeppener, S.; D’Haese, C.; Kempe, K.; Mansfeld, U.; Paulus, R.M.; Gohy, J.F.; Schubert, U.S.; Hoogenboom, R. Self-assembly of chiral block and gradient copolymers. Soft Matter 2012, 8, 165–172. [Google Scholar] [CrossRef]
- Guo, X.Q.; Schulz, R.C. Synthesis and characterization of optically active 2,4-disubstituted-2-oxazolines and their polymerization. Polym. Int. 1994, 34, 229–233. [Google Scholar] [CrossRef]
- Mendrek, B.; Fus-Kujawa, A.; Teper, P.; Botor, M.; Kubacki, J.; Sieroń, A.L.; Kowalczuk, A. Star polymer-based nanolayers with immobilized complexes of polycationic stars and DNA for deposition gene delivery and recovery of intact transfected cells. Int. J. Pharm. 2020, 589, 119823. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, B.; Fus, A.; Klarzyńska, K.; Sieroń, A.L.; Smet, M.; Kowalczuk, A.; Dworak, A. Synthesis, characterization and cytotoxicity of novel thermoresponsive star copolymers of N,N0-dimethylaminoethyl methacrylate and hydroxyl-bearing oligo(ethylene glycol) methacrylate. Polymers 2018, 10, 1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloksma, M.M.; Bakker, D.J.; Weber, C.; Hoogenboom, R.; Schubert, U.S. The effect of hofmeister salts on the LCST transition of poly(2-oxazoline)s with varying hydrophilicity. Macromol. Rapid Commun. 2010, 31, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.; Ernoch, P.; Zenke, I.; Runge, H.; Pitschke, R.; Hartmann, J.; Tiersch, B.; Schlaad, H. Mechanistic study of the phase separation/crystallization process of poly(2-isopropyl-2-oxazoline) in hot water. Soft Matter 2010, 6, 3784–3788. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Cyclische Imidsäureester aus Nitrilen und Aminoalkoholen. Justus Liebigs Ann. Chem. 1974, 1974, 996–1009. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wałach, W.; Klama-Baryła, A.; Sitkowska, A.; Kowalczuk, A.; Oleszko-Torbus, N. Alternative to Poly(2-isopropyl-2-oxazoline) with a Reduced Ability to Crystallize and Physiological LCST. Int. J. Mol. Sci. 2021, 22, 2221. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042221
Wałach W, Klama-Baryła A, Sitkowska A, Kowalczuk A, Oleszko-Torbus N. Alternative to Poly(2-isopropyl-2-oxazoline) with a Reduced Ability to Crystallize and Physiological LCST. International Journal of Molecular Sciences. 2021; 22(4):2221. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042221
Chicago/Turabian StyleWałach, Wojciech, Agnieszka Klama-Baryła, Anna Sitkowska, Agnieszka Kowalczuk, and Natalia Oleszko-Torbus. 2021. "Alternative to Poly(2-isopropyl-2-oxazoline) with a Reduced Ability to Crystallize and Physiological LCST" International Journal of Molecular Sciences 22, no. 4: 2221. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042221