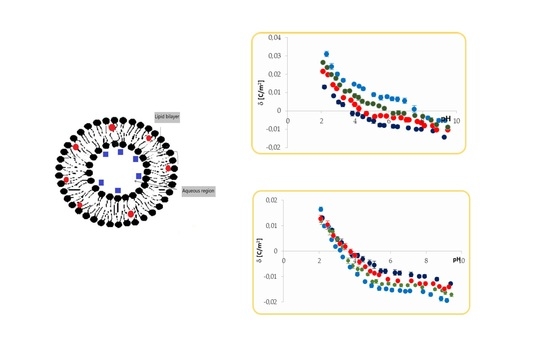
To explore the interactions between charged drugs—perphenazine or barbituric acid and neutral lipid membranes—a series of microelectrophoretic experiments were carried out. These interactions can potentially change liposome electrical properties; therefore, membrane surface charge densities were determined using the ELS technique. All measurements were performed as a function of pH.
3.1. Effect of Perphenazine on Surface Charge Densities of Neutral Phosphatidylcholine Liposomal Membranes
Electrophoretic mobility measurements were made for pure
PC and
PC/
PF 30:1; 20:1; 10:1 liposomes. The pH dependences of the surface charge densities determined from electrophoretic mobility data (Equation (28)) are given in
Figure 2. Representative plots from at least three independent measurements for each of the membrane systems are presented.
As shown in the figure, the solution pH and membrane composition have a significant effect on the surface charge of all analyzed systems. If we consider the curve obtained for a pure
PC, we notice that the isoelectric point (IEP)—one of the most critical parameters describing variable-charge surfaces—was found to be at pH ~ 4, and the surface charge density increases (in absolute value) as the pH moves away from the IEP. As solution pH varies, differential concentrations of cations (protons and sodium ions)/anions (hydroxide and chloride ions) act as counterions to neutralize either phosphate or choline groups on
PC lipids, thus potentially altering the surface charge [
21]. For pH < 4,
PC membranes exhibit positive surface charge density values indicating cations’ binding with the membrane surface. At pH > 4, the membranes exhibit negative surface charge density values indicating anions’ association (mainly hydroxide ions) to the membrane surface. If we consider curves obtained for perphenazine-modified liposomal membranes, we observe that the drug’s presence results in a considerable increase in surface charge densities in the whole tested pH range (pH 2–9.5). The results give evidence that perphenazine significantly influences a shift in the IEP of the
PC membrane, from pH ~ 4 for pure
PC to pH ~ 7.2 for 10:1
PC/PF membranes (
Table 1). As additional data, to better clarify the obtained surface charge density results, analyzed liposomes’ electrophoretic mobilities are presented in
Table 2.
The presence of perphenazine causes a change in the magnitude and even a sign of the phosphatidylcholine membrane surface charge density, and the changes are as expected. The drug is positively charged under physiological conditions, therefore its presence in the zwitterionic lipid membrane should increase its surface charge. In acidic pH, an increase in the positive charge of PF-treated membranes compared to the pure PC membrane is observed. This is caused by the shielding of the negative groups of PC molecules by protons coupled with exposure of the compounds’ positively charged groups and the association of chloride ions with these groups. In basic solutions, we observe more of a decrease in the negative charge of PF-treated membranes than pure PC membrane and a shift of the IEP to high pH values. The positive groups of PC and PF are shielded by hydroxide ions, while the negative groups of PC are exposed and are associated with sodium ions.
Theoretical surface charge density values for the
PC/
PF liposomal system were determined by applying Equation (12) to the experimental data (the values for the
PC membrane we determined previously [
27]). Reported values were used to obtain numeric values of the association constants of the positive species of
PF with
OH− and
Cl− ions from Equations (13) and (14) (
Table 3). Then, after substituting them into Equation (12), theoretical data were obtained. To compare the experimental and theoretical surface charge densities vs. pH of the
PC/
PF 10:1 liposomal membrane, they are plotted on one graph (
Figure 3). Due to a lack of legibility, data for
PC/
PF 30:1 and
PC/
PF 20:1 membranes are not included in the figure, but good fits have also been obtained for these systems.
As shown from the figure, the theoretical curve matches the experimental point in the whole pH range quite well, although it is not perfect coverage. These differences are most likely because the model describing the
PC/
PF membrane system does not consider interactions between the lipid and the drug, only the association of electrolyte ions to the membrane’s surface. We have attempted to extend the model (
Section 2.1.) with an additional equilibrium considering a complex formation of the lipid and the drug. However, such a large number of equilibria resulted in a significant complication of the model and made it impossible to determine the searching parameters.
3.2. Effect of Barbituric Acid on Surface Charge Densities of Neutral Phosphatidylcholine Liposomal Membranes
The pH dependences of the surface charge densities for pure
PC and
PC/
BA 30:1; 20:1; 10:1 liposomal membranes are presented in
Figure 4. Representative plots from at least three independent measurements for each of the membrane systems are shown.
Figure 4 and
Table 4 show that similarly to the
PC/
PF system (
Figure 2), in the case of the
PC/
BA system, both pH and membrane composition influence the IEP position and surface charge density of all analyzed systems. It can also be noticed that the profiles of all four curves are the same. The IEP slightly shifts towards acidic pH values as the
BA content in the membrane increases (from pH ~ 4 for pure
PC to pH ~ 3.2 for 10:1
PC/
BA membranes). For pH < 3, there are no statistically significant changes in surface charge values of all analyzed membranes. This is because, in acidic pH, negative
PC and
BA species are shielded by protons, whereas positively charged
PC groups are exposed and chloride ions bind to them. However, there is a noticeable difference between the membrane surface charge densities (including standard deviations) in the pH range between 3 and 9. Barbituric acid-modified phosphatidylcholine membranes exhibit a more negative surface charge compared to unmodified ones. These changes are more significant the higher the content of the negatively charged drug in the neutral phosphatidylcholine membrane. In basic solutions, we observe an increase in the negative charge of
BA-treated membranes compared to the pure
PC membrane. It is caused by the positive groups of
PC, which are covered by hydroxide ions while the negative species of both
PC and
BA are exposed and are associated with sodium ions. Additionally, such as in
PC/
PF systems, electrophoretic mobility values of the liposomes are presented in
Table 5.
The
PC/
BA liposomal system’s theoretical surface charge density data were determined by applying Equation (25) to the experimental data. Previously determined [
27] association constants of solution ions with
PC groups (
were used to obtain association constants of the negative species of
BA with H
+ and Na
+ ions (
) from Equations (26) and (27) (
Table 6). Then, after substituting them into Equation (25), theoretical surface charge density was calculated. A comparison of experimental data and theoretical values obtained for the
PC/
BA 20:1 liposomal membrane is presented in
Figure 5. For the graph clarity, data for the other two
BA-modified membranes were not included in it; however, a good fit of results for these systems was also obtained.
It can be seen from the figure that the experimental points are in good agreement with the theoretical values in the range of pH 2.5–8. It is not easy to indicate precisely which interactions cause the incompatibility at other pH values; we may suppose the cause is due to the drug–lipid complex formation. Assumptions of each of the two proposed mathematical models describing interactions in the analyzed systems (
Section 2.1 and
Section 2.2) are, in our view, correct; however, they require an improvement. In the models, we considered the adsorption of solution ions on cationic/anionic membrane surfaces only. Undoubtedly, each of the models needs to be expanded with the equilibrium between phosphatidylcholine and perphenazine/barbituric acid. Unfortunately, the additional equilibrium complicates the models enormously because of the presence of a large number of parameters characterizing the equilibria. Therefore, it is necessary to adopt certain simplifications to reduce the number of these parameters—only then will it be possible to design all the searched values. However, despite numerous attempts, we were unable to do so.
Additionally, we measured the sizes of
PC,
PC/
PF, and
PC/
BA liposomes (pH = 7.4). The dynamic light scattering data are presented in
Table 7.
It can be seen that pure
PC liposomes and
PC liposomes modified by both CNS drugs exhibit bimodal particle size distribution profiles and a polydispersity index (PDI) in the range between 0.294 to 0.421, indicating that analyzed liposomes are polydisperse. An example size distribution graph by the intensity of scattered light is shown in
Figure 6. An increase in the particle size of liposomes containing both
PF and
BA compared to pure
PC liposomes indicating that the drugs’ proportion in the formulations also influences the liposome diameter should also be noticed. Observed dependencies can be attributed to the fact that the drugs are ionized in physiological pH and interact on the lipid membrane surface.
The structure and biophysical characteristics of cell membranes may define whether and how they will respond to the drug binding. In turn, the drug’s properties may also modulate its position and conformation within biomembranes. For these reasons, investigations of drug binding to membranes become crucial to understand multi-drug resistance mechanisms or the development of undesirable side effects [
28]. Our research focused on the interaction of both cationic and anionic molecules with zwitterionic lipid membranes. We selected the cationic perphenazine partly because such drugs are commonly associated with overdose and partly because they (similarly to other phenothiazines) influence electrical properties of lipid membranes. Hidalgo investigated the effect of trifluoperazine and chlorpromazine on lipid monolayers and found that binding of the drugs induces changes in surface pressure and surface potential [
29]. We choose the anionic barbituric acid because it is a compound of high pharmacological importance. However, there are a lack of studies concerning barbituric acid interactions with membrane components; only data for barbiturates, such as phenobarbital or pentobarbital, can be found [
30].
Membrane composition and solution pH affect its surface charge. The parameter changes are due to the modification of phosphatidylcholine membranes with the drugs acting on the central nervous system. These changes can be attributed to interactions between the membrane components and the environment and between the membrane components (lipid and drug). We want to emphasize that, to the best of our knowledge, no published study in the literature has considered interactions of the drugs with lipid membranes as a function of pH. We hope that the experimental data and determining association constants may help develop a better understanding of the influence of charged drugs on phospholipid membranes’ electrical properties. It should be emphasized that examining phenomena in which cell membranes participate and their interaction with drugs both in physiological conditions and caused or associated with particular disease states that it is crucial to exploit the molecular bases of many diseases and identify new treatment strategies.