Nox2 Inhibition Regulates Stress Response and Mitigates Skeletal Muscle Fiber Atrophy during Simulated Microgravity
Abstract
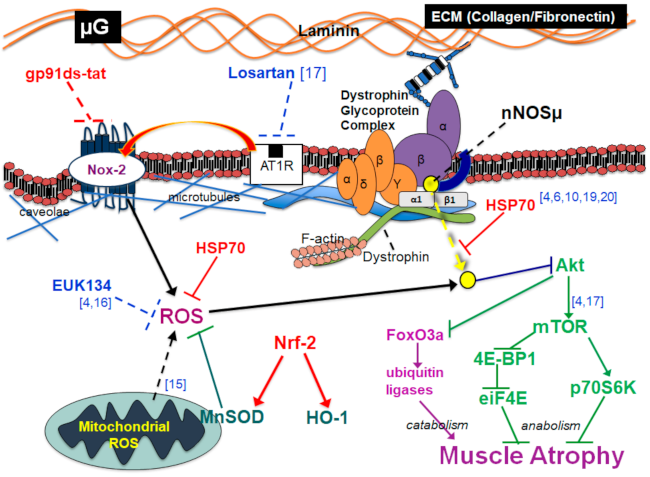
:1. Introduction
2. Results
2.1. Effect of Nox2 Inhibition on the Nox2 Complex Assembly
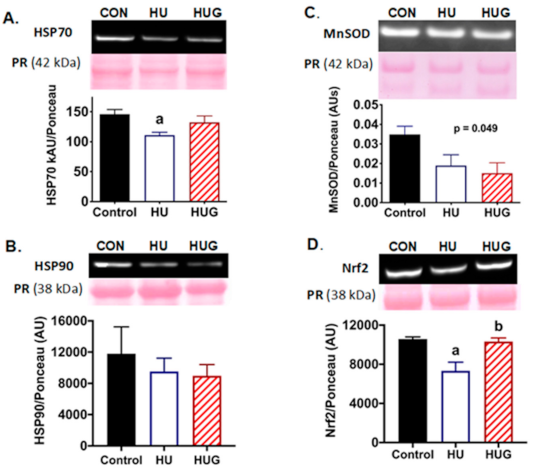
2.2. Nox2 Inhibition Attenuates Disruption of HSP70 Protein Expression
2.3. Nrf2 Levels Are Elevated by the Inhibition of Nox2
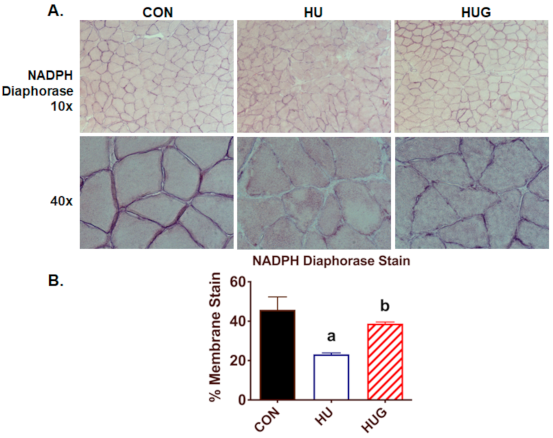
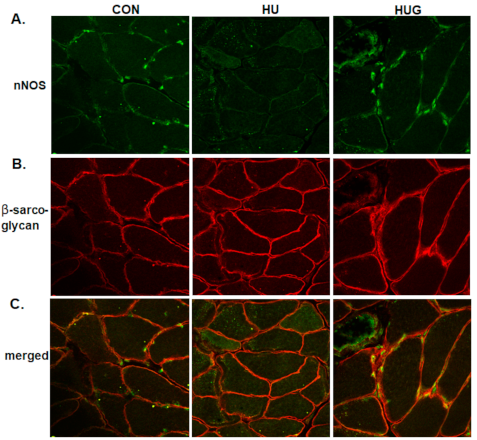
2.4. Nox2 Inhibition Mitigates the Reduction in nNOS Activity and Translocation
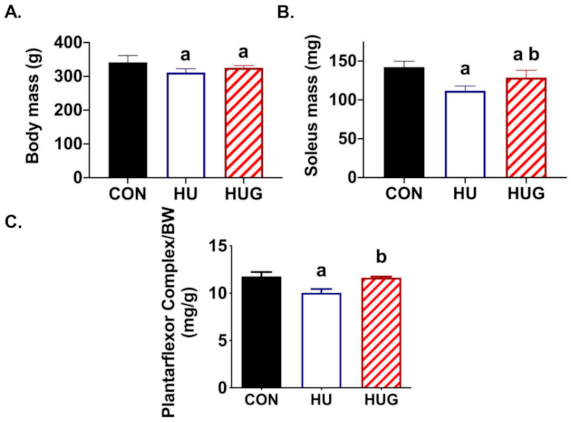
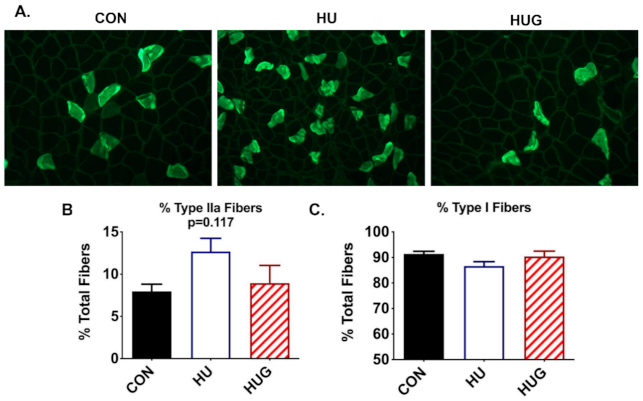
2.5. Changes in Skeletal Muscle Morphology
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Design and Hindlimb Unloading
4.3. Muscle Tissue Preparation
4.4. Western Immunoblot
4.5. Histological Analysis
4.6. Immunofluorescence (IF)
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fitts, R.H.; Romatowski, J.G.; Peters, J.R.; Paddon-Jones, D.; Wolfe, R.R.; Ferrando, A.A. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am. J. Physiology. Cell Physiol. 2007, 293, C313–C320. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Roy, R.R.; Allen, D.L.; Monti, R.J. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am. J. Phys. Med. Rehabil. 2002, 81 (Suppl. 11), S127–S147. [Google Scholar] [CrossRef]
- Arbogast, S.; Smith, J.; Matuszczak, Y.; Hardin, B.J.; Moylan, J.S.; Smith, J.D.; Ware, J.; Kennedy, A.R.; Reid, M.B. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J. Appl. Physiol. 2007, 102, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Kuczmarski, J.M.; Hord, J.M.; Lee, Y.; Guzzoni, V.; Rodriguez, D.; Lawler, M.S.; Garcia-Villatoro, E.L.; Holly, D.; Ryan, P.; Falcon, K.; et al. Effect of Eukarion-134 on Akt-mTOR signalling in the rat soleus during 7 days of mechanical unloading. Exp. Physiol. 2018, 103, 545–558. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.; Judge, A. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senf, S.M.; Dodd, S.L.; McClung, J.M.; Judge, A.R. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008, 22, 3836–3845. [Google Scholar] [CrossRef]
- Lawler, J.M.; Kwak, H.-B.; Kim, J.-H.; Lee, Y.; Hord, J.M.; Martinez, D.A. Biphasic stress response in the soleus during reloading after hind limb unloading. Med. Sci. Sports Exerc. 2012, 44, 600–609. [Google Scholar] [CrossRef]
- Lawler, J.M.; Song, W.; Kwak, H.B. Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 200–207. [Google Scholar] [CrossRef]
- Senf, S.M.; Dodd, S.L.; Judge, A.R. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell Physiol. 2010, 298, C38–C45. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J.M.; Garcia-Villatoro, E.L.; Guzzoni, V.; Hord, J.M.; Botchlett, R.; Holly, D.; Lawler, M.S.; Janini Gomes, M.; Ryan, P.; Rodriguez, D.; et al. Effect of combined fish oil & Curcumin on murine skeletal muscle morphology and stress response proteins during mechanical unloading. Nutr. Res. 2019, 65, 17–28. [Google Scholar] [PubMed]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef]
- Koesterer, T.; Dodd, S.L.; Powers, S. Increased antioxidant capacity does not attenuate muscle atrophy caused by unweighting. J. Appl. Physiol. 2002, 93, 1959–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farid, M.; Reid, M.B.; Li, Y.P.; Gerken, E.; Durham, W.J. Effects of dietary curcumin or N-acetylcysteine on NF-kappaB activity and contractile performance in ambulatory and unloaded murine soleus. Nutr. Metab. 2005, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Dodd, S.L.; Gagnon, B.J.; Senf, S.M.; Hain, B.A.; Judge, A.R. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve 2010, 41, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Smuder, A.J.; Kwon, O.-s.; Kavazis, A.N.; Szeto, H.H.; Powers, S.K. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J. Appl. Physiol. 2011, 111, 1459–1466. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J.M.; Kunst, M.; Hord, J.M.; Lee, Y.; Joshi, K.; Botchlett, R.E.; Ramirez, A.; Martinez, D.A. EUK-134 ameliorates nNOSμ translocation and skeletal muscle fiber atrophy during short-term mechanical unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R470–R482. [Google Scholar] [CrossRef]
- Hord, J.M.; Garcia, M.M.; Farris, K.R.; Guzzoni, V.; Lee, Y.; Lawler, M.S.; Lawler, J.M. Nox2 signaling and muscle fiber remodeling are attenuated by losartan administration during skeletal muscle unloading. Physiol. Rep. 2021, 9, e14606. [Google Scholar] [CrossRef]
- Rey, F.; Cifuentes, M.; Kiarash, A.; Quinn, M.; Pagano, P. Novel competitive inhibitor of NAD (P) H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ. Res. 2001, 89, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Motohashi, N.; Uezumi, A.; Fukada, S.-I.; Yoshimura, T.; Itoyama, Y.; Aoki, M.; Miyagoe-Suzuki, Y.; Takeda, S.I. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J. Clin. Investig. 2007, 117, 2468–2476. [Google Scholar] [CrossRef] [Green Version]
- Vitadello, M.; Gherardini, J.; Gorza, L. The stress protein/chaperone Grp94 counteracts muscle disuse atrophy by stabilizing subsarcolemmal neuronal nitric oxide synthase. Antioxid. Redox Signal. 2014, 20, 2479–2496. [Google Scholar] [CrossRef] [Green Version]
- da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Naito, H.; Powers, S.K.; Demirel, H.A.; Sugiura, T.; Dodd, S.L.; Aoki, J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J. Appl. Physiol. 2000, 88, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Lechado, A.I.T.; Vitadello, M.; Traini, L.; Namuduri, A.V.; Gastaldello, S.; Gorza, L. Sarcolemmal loss of active nNOS (Nos1) is an oxidative stress-dependent, early event driving disuse atrophy. J. Pathol. 2018, 246, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Germinario, E.; Ravara, B.; Libera, L.D.; Danieli-Betto, D.; Gorza, L. Curcumin counteracts loss of force and atrophy of hindlimb unloaded rat soleus by hampering neuronal nitric oxide synthase untethering from sarcolemma. J. Physiol. 2014, 592, 2637–2652. [Google Scholar] [CrossRef]
- McArdle, A.; Dillmann, W.H.; Mestril, R.; Faulkner, J.A.; Jackson, M.J. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 355–357. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase (s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.W.; Prosser, B.L.; Lederer, W.J. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid. Redox Signal. 2014, 20, 929–936. [Google Scholar] [CrossRef]
- Lawler, J.M.; Cline, C.C.; Hu, Z.; Coast, J.R. Effect of oxidant challenge on contractile function of the aging rat diaphragm. Am. J. Physiol. 1997, 272 (2 Pt 1), E201–E207. [Google Scholar] [CrossRef]
- Lawler, J.M.; Kim, J.-H.; Kwak, H.-B.; Barnes, W.S. Redox modulation of diaphragm contractility: Interaction between DHPR and RyR channels. Free Radic. Biol. Med. 2010, 49, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Henríquez-Olguín, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Merry, T.L.; Steinberg, G.R.; Lynch, G.S.; McConell, G.K. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E577–E585. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Rodriguez, D.A.; Hord, J.M. Mitochondria in the middle: Exercise preconditioning protection of striated muscle. J. Physiol. 2016, 594, 5161–5183. [Google Scholar] [CrossRef] [Green Version]
- Henríquez-Olguín, C.; Boronat, S.; Cabello-Verrugio, C.; Jaimovich, E.; Hidalgo, E.; Jensen, T.E. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxid. Redox Signal. 2019, 31, 1371–1410. [Google Scholar] [CrossRef]
- Henríquez-Olguín, C.; Díaz-Vegas, A.; Utreras-Mendoza, Y.; Campos, C.; Arias-Calderón, M.; Llanos, P.; Contreras-Ferrat, A.; Espinosa, A.; Altamirano, F.; Jaimovich, E. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front. Physiol. 2016, 7, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosser, B.L.; Ward, C.W.; Lederer, W. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science 2011, 333, 1440–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto Padron de Figueiredo, A.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef] [Green Version]
- Scalabrin, M.; Pollock, N.; Staunton, C.A.; Brooks, S.V.; McArdle, A.; Jackson, M.J.; Vasilaki, A. Redox responses in skeletal muscle following denervation. Redox Biol. 2019, 26, 101294. [Google Scholar] [CrossRef] [PubMed]
- Costa-Beber, L.C.; Hirsch, G.E.; Heck, T.G.; Ludwig, M.S. Chaperone duality: The role of extracellular and intracellular HSP70 as a biomarker of endothelial dysfunction in the development of atherosclerosis. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Lorenzo, A.F.G.; Costantino, V.V.; Appiolaza, M.L.; Cacciamani, V.; Benardon, M.E.; Bocanegra, V.; Vallés, P.G. Heat shock protein 70 and CHIP promote Nox4 ubiquitination and degradation within the losartan antioxidative effect in proximal tubule cells. Cell. Physiol. Biochem. 2015, 36, 2183–2197. [Google Scholar] [CrossRef]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef]
- Chazarin, B.; Ziemianin, A.; Evans, A.L.; Meugnier, E.; Loizon, E.; Chery, I.; Arnemo, J.M.; Swenson, J.E.; Gauquelin-Koch, G.; Simon, C. Limited oxidative stress favors resistance to skeletal muscle atrophy in hibernating brown bears (Ursus arctos). Antioxidants 2019, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.D.; Fan, S.D.; Chen, X.Y.; Yan, X.L.; Zhang, X.Z.; Ma, B.W.; Yu, D.Y.; Xiao, W.Y.; Zhuang, C.L.; Yu, Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Takeda, K.; Tamura, Y.; Fujimaki, S.; Takemasa, T.; Hatta, H. Nrf2 deficiency does not affect denervation-induced alterations in mitochondrial fission and fusion proteins in skeletal muscle. Physiol. Rep. 2016, 4, e13064. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, C.S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food 2018, 21, 551–559. [Google Scholar] [CrossRef]
- Bozaykut, P.; Ozer, N.K.; Karademir, B. Nrf2 silencing to inhibit proteolytic defense induced by hyperthermia in HT22 cells. Redox Biol. 2016, 8, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.-J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef]
- Naidu, S.; Vijayan, V.; Santoso, S.; Kietzmann, T.; Immenschuh, S. Inhibition and Genetic Deficiency of p38 MAPK Up-Regulates Heme Oxygenase-1 Gene Expression via Nrf2. J. Immunol. 2009, 182, 7048–7057. [Google Scholar] [CrossRef] [Green Version]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [Green Version]
- Fitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.M.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588 Pt 18, 3567–3592. [Google Scholar] [CrossRef]
- Dimauro, I.; Pearson, T.; Caporossi, D.; Jackson, M.J. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res. Notes 2012, 5, 513. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawler, J.M.; Hord, J.M.; Ryan, P.; Holly, D.; Janini Gomes, M.; Rodriguez, D.; Guzzoni, V.; Garcia-Villatoro, E.; Green, C.; Lee, Y.; et al. Nox2 Inhibition Regulates Stress Response and Mitigates Skeletal Muscle Fiber Atrophy during Simulated Microgravity. Int. J. Mol. Sci. 2021, 22, 3252. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22063252
Lawler JM, Hord JM, Ryan P, Holly D, Janini Gomes M, Rodriguez D, Guzzoni V, Garcia-Villatoro E, Green C, Lee Y, et al. Nox2 Inhibition Regulates Stress Response and Mitigates Skeletal Muscle Fiber Atrophy during Simulated Microgravity. International Journal of Molecular Sciences. 2021; 22(6):3252. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22063252
Chicago/Turabian StyleLawler, John M., Jeffrey M. Hord, Pat Ryan, Dylan Holly, Mariana Janini Gomes, Dinah Rodriguez, Vinicius Guzzoni, Erika Garcia-Villatoro, Chase Green, Yang Lee, and et al. 2021. "Nox2 Inhibition Regulates Stress Response and Mitigates Skeletal Muscle Fiber Atrophy during Simulated Microgravity" International Journal of Molecular Sciences 22, no. 6: 3252. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22063252