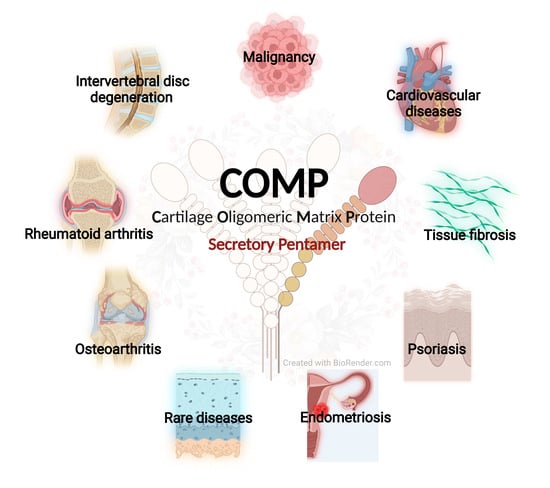
Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities
Abstract
:1. Introduction
2. Overview of COMP
3. COMP and Skeleton Diseases
3.1. COMP and Cartilage Homeostasis
3.2. COMP Mutations and Skeleton Diseases
3.3. The Use of COMP as a Biomarker for OA, RA, and Other Skeleton Diseases
3.4. Treatments and Targeting COMP in OA and Other Pathologies
4. COMP and Cancer/Cancer Malignancy/Cancer Progression
4.1. Hepatocellular Carcinoma
4.2. Colon Cancer
4.3. Breast Cancer
4.4. Others
5. COMP and Cardiovascular Disease
6. COMP and Fibrosis
7. COMP and Other Diseases
8. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maly, K.; Andres Sastre, E.; Farrell, E.; Meurer, A.; Zaucke, F. COMP and TSP-4: Functional Roles in Articular Cartilage and Relevance in Osteoarthritis. Int. J. Mol. Sci 2021, 22, 2242. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Tatari, H. The structure, physiology, and biomechanics of articular cartilage: Injury and repair. Acta Orthop. Traumatol. Turc. 2007, 41 (Suppl. S2), 1–5. [Google Scholar] [PubMed]
- Hua, R.; Ni, Q.; Eliason, T.D.; Han, Y.; Gu, S.; Nicolella, D.P.; Wang, X.; Jiang, J.X. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Biol. 2020, 94, 95–109. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Huang, Y.; Gu, S.; Jiang, J.X. Coupling Effect of Water and Proteoglycans on the In Situ Toughness of Bone. J. Bone Miner. Res. 2016, 31, 1026–1029. [Google Scholar] [CrossRef]
- Wang, X.; Hua, R.; Ahsan, A.; Ni, Q.; Huang, Y.; Gu, S.; Jiang, J.X. Age-Related Deterioration of Bone Toughness Is Related to Diminishing Amount of Matrix Glycosaminoglycans (Gags). JBMR Plus 2018, 2, 164–173. [Google Scholar] [CrossRef]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef]
- Tan, K.; Lawler, J. The interaction of Thrombospondins with extracellular matrix proteins. J. Cell Commun. Signal. 2009, 3, 177–187. [Google Scholar] [CrossRef]
- Lopez-Franco, M.; Lopez-Franco, O.; Murciano-Anton, M.A.; Canamero-Vaquero, M.; Fernandez-Acenero, M.J.; Herrero-Beaumont, G.; Gomez-Barrena, E. Meniscal degeneration in human knee osteoarthritis: In situ hybridization and immunohistochemistry study. Arch. Orthop. Trauma. Surg. 2016, 136, 175–183. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawabata, K.; Kusaka-Kikushima, A.; Sugiyama, Y.; Mabuchi, T.; Takekoshi, S.; Miyasaka, M.; Ozawa, A.; Sakai, S. Cartilage Oligomeric Matrix Protein Increases in Photodamaged Skin. J. Investig. Dermatol. 2016, 136, 1143–1149. [Google Scholar] [CrossRef]
- Liang, Y.; Fu, Y.; Qi, R.; Wang, M.; Yang, N.; He, L.; Yu, F.; Zhang, J.; Yun, C.H.; Wang, X.; et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood 2015, 126, 905–914. [Google Scholar] [CrossRef] [PubMed]
- El Defrawy, A.O.; Gheita, T.A.; Raslan, H.M.; El Ansary, M.M.; El Awar, A.H. Serum and synovial cartilage oligomeric matrix protein levels in early and established rheumatoid arthritis. Z Rheumatol. 2016, 75, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Hauser, N.; Paulsson, M.; DiCesare, P.E.; Michel, B.A.; Hauselmann, H.J. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br. J. Rheumatol. 1997, 36, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Koelling, S.; Clauditz, T.S.; Kaste, M.; Miosge, N. Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, R56. [Google Scholar] [CrossRef] [PubMed]
- Coustry, F.; Posey, K.L.; Maerz, T.; Baker, K.; Abraham, A.M.; Ambrose, C.G.; Nobakhti, S.; Shefelbine, S.J.; Bi, X.; Newton, M.; et al. Mutant cartilage oligomeric matrix protein (COMP) compromises bone integrity, joint function and the balance between adipogenesis and osteogenesis. Matrix Biol. 2018, 67, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Hoffman, S.M.; King, L.M.; Olsen, A.S.; Mohrenweiser, H.; Leroy, J.G.; Mortier, G.R.; Rimoin, D.L.; Lachman, R.S.; Gaines, E.S.; et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat. Genet. 1995, 10, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Kempta Lekpa, F.; Piette, J.C.; Bastuji-Garin, S.; Kraus, V.B.; Stabler, T.V.; Poole, A.R.; Marini-Portugal, A.; Chevalier, X. Serum cartilage oligomeric matrix protein (COMP) level is a marker of disease activity in relapsing polychondritis. Clin. Exp. Rheumatol. 2010, 28, 553–555. [Google Scholar] [PubMed]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Hou, H.; Liu, T.; Su, S.; Xi, X.; Liao, Y.; Xie, R.; Jin, G.; Liu, X.; Zhu, L.; et al. Cartilage Oligomeric Matrix Protein promotes epithelial-mesenchymal transition by interacting with Transgelin in Colorectal Cancer. Theranostics 2020, 10, 8790–8806. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, K.S.; Hagerling, C.; Ryden, L.; Larsson, A.M.; Blom, A.M. High Levels of Expression of Cartilage Oligomeric Matrix Protein in Lymph Node Metastases in Breast Cancer Are Associated with Reduced Survival. Cancers 2021, 13, 5876. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Hu, W.P.; Xie, L.; Xiang, G.L.; Wu, Q.H.; Qu, J.M.; Li, S.Q.; Guan, L.H.; Liu, D. Serum cartilage oligomeric matrix protein is decreased in patients with pulmonary hypertension: A potential protective factor. Pulm. Circ. 2021, 11, 0271678X20978861. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Wang, B.; Li, B.; Lv, H.; He, J.; Huang, Y.; Cui, Z.; Ma, Q.; Li, T.; et al. COMP (Cartilage Oligomeric Matrix Protein), a Novel PIEZO1 Regulator That Controls Blood Pressure. Hypertension 2022, 79, 549–561. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, Y.; Yang, Z.; Chen, Y.; Zheng, J.; Mao, C.; Li, Z.; Liu, Z.; Yu, B.; Li, T.; et al. Cartilage oligomeric matrix protein is an endogenous beta-arrestin-2-selective allosteric modulator of AT1 receptor counteracting vascular injury. Cell Res. 2021, 31, 773–790. [Google Scholar] [CrossRef]
- Schulz, J.N.; Plomann, M.; Sengle, G.; Gullberg, D.; Krieg, T.; Eckes, B. New developments on skin fibrosis—Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol. 2018, 68–69, 522–532. [Google Scholar] [CrossRef]
- Moon, S.J.; Bae, J.M.; Park, K.S.; Tagkopoulos, I.; Kim, K.J. Compendium of skin molecular signatures identifies key pathological features associated with fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 817–825. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Zachou, K.; Giannoulis, G.; Gabeta, S.; Norman, G.L.; Dalekos, G.N. Serum Cartilage Oligomeric Matrix Protein and Golgi Protein-73: New Diagnostic and Predictive Tools for Liver Fibrosis and Hepatocellular Cancer? Cancers 2021, 13, 3510. [Google Scholar] [CrossRef]
- Acharya, C.; Yik, J.H.; Kishore, A.; Van Dinh, V.; Di Cesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol. 2014, 37, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C.; Lawler, J. The thrombospondins. Cold Spring Harb. Perspect Biol. 2011, 3, a009712. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Duquette, M.; Joachimiak, A.; Lawler, J. The crystal structure of the signature domain of cartilage oligomeric matrix protein: Implications for collagen, glycosaminoglycan and integrin binding. FASEB J. 2009, 23, 2490–2501. [Google Scholar] [CrossRef] [PubMed]
- Thur, J.; Rosenberg, K.; Nitsche, D.P.; Pihlajamaa, T.; Ala-Kokko, L.; Heinegard, D.; Paulsson, M.; Maurer, P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I., II, and IX. J. Biol. Chem. 2001, 276, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.N.; Nuchel, J.; Niehoff, A.; Bloch, W.; Schonborn, K.; Hayashi, S.; Kamper, M.; Brinckmann, J.; Plomann, M.; Paulsson, M.; et al. COMP-assisted collagen secretion--a novel intracellular function required for fibrosis. J. Cell Sci. 2016, 129, 706–716. [Google Scholar] [CrossRef]
- Agarwal, P.; Zwolanek, D.; Keene, D.R.; Schulz, J.N.; Blumbach, K.; Heinegard, D.; Zaucke, F.; Paulsson, M.; Krieg, T.; Koch, M.; et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem. 2012, 287, 22549–22559. [Google Scholar] [CrossRef]
- Chen, F.H.; Herndon, M.E.; Patel, N.; Hecht, J.T.; Tuan, R.S.; Lawler, J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J. Biol. Chem. 2007, 282, 24591–24598. [Google Scholar] [CrossRef]
- Di Cesare, P.E.; Chen, F.S.; Moergelin, M.; Carlson, C.S.; Leslie, M.P.; Perris, R.; Fang, C. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002, 21, 461–470. [Google Scholar] [CrossRef]
- Ishida, K.; Acharya, C.; Christiansen, B.A.; Yik, J.H.; DiCesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 2013, 55, 23–35. [Google Scholar] [CrossRef]
- Halasz, K.; Kassner, A.; Morgelin, M.; Heinegard, D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 2007, 282, 31166–31173. [Google Scholar] [CrossRef]
- Haudenschild, D.R.; Hong, E.; Yik, J.H.; Chromy, B.; Morgelin, M.; Snow, K.D.; Acharya, C.; Takada, Y.; Di Cesare, P.E. Enhanced activity of transforming growth factor beta1 (TGF-beta1) bound to cartilage oligomeric matrix protein. J. Biol. Chem. 2011, 286, 43250–43258. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.J.; Janssen, M.P.F.; Peeters, L.; Haudenschild, D.R.; Cremers, A.; Surtel, D.A.M.; van Rhijn, L.W.; Emans, P.J.; Welting, T.J.M. Aggrecan and COMP Improve Periosteal Chondrogenesis by Delaying Chondrocyte Hypertrophic Maturation. Front. Bioeng. Biotechnol. 2020, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Unger, S.; Hecht, J.T. Pseudoachondroplasia and multiple epiphyseal dysplasia: New etiologic developments. Am. J. Med. Genet. 2001, 106, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Dinser, R.; Zaucke, F.; Kreppel, F.; Hultenby, K.; Kochanek, S.; Paulsson, M.; Maurer, P. Pseudoachondroplasia is caused through both intra- and extracellular pathogenic pathways. J. Clin. Investig. 2002, 110, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Brock, J.; Ramsden, S.C.; Bell, P.A. Genotype to phenotype correlations in cartilage oligomeric matrix protein associated chondrodysplasias. Eur. J. Hum. Genet. 2014, 22, 1278–1282. [Google Scholar] [CrossRef]
- Kennedy, J.; Jackson, G.; Ramsden, S.; Taylor, J.; Newman, W.; Wright, M.J.; Donnai, D.; Elles, R.; Briggs, M.D. COMP mutation screening as an aid for the clinical diagnosis and counselling of patients with a suspected diagnosis of pseudoachondroplasia or multiple epiphyseal dysplasia. Eur. J. Hum. Genet. 2005, 13, 547–555. [Google Scholar] [CrossRef]
- Suleman, F.; Gualeni, B.; Gregson, H.J.; Leighton, M.P.; Pirog, K.A.; Edwards, S.; Holden, P.; Boot-Handford, R.P.; Briggs, M.D. A novel form of chondrocyte stress is triggered by a COMP mutation causing pseudoachondroplasia. Hum. Mutat. 2012, 33, 218–231. [Google Scholar] [CrossRef]
- Posey, K.L.; Veerisetty, A.C.; Liu, P.; Wang, H.R.; Poindexter, B.J.; Bick, R.; Alcorn, J.L.; Hecht, J.T. An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am. J. Pathol. 2009, 175, 1555–1563. [Google Scholar] [CrossRef]
- Coustry, F.; Posey, K.L.; Liu, P.; Alcorn, J.L.; Hecht, J.T. D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am. J. Pathol. 2012, 180, 738–748. [Google Scholar] [CrossRef]
- Posey, K.L.; Coustry, F.; Veerisetty, A.C.; Liu, P.; Alcorn, J.L.; Hecht, J.T. Chop (Ddit3) is essential for D469del-COMP retention and cell death in chondrocytes in an inducible transgenic mouse model of pseudoachondroplasia. Am. J. Pathol. 2012, 180, 727–737. [Google Scholar] [CrossRef]
- Liang, H.; Hou, Y.; Pang, Q.; Jiang, Y.; Wang, O.; Li, M.; Xing, X.; Zhu, H.; Xia, W. Clinical, Biochemical, Radiological, Genetic and Therapeutic Analysis of Patients with COMP Gene Variants. Calcif. Tissue Int. 2022, 110, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.L.; Coustry, F.; Veerisetty, A.C.; Liu, P.; Alcorn, J.L.; Hecht, J.T. Chondrocyte-specific pathology during skeletal growth and therapeutics in a murine model of pseudoachondroplasia. J. Bone Miner. Res. 2014, 29, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Veerisetty, A.C.; Hossain, M.G.; Patra, D.; Chiu, F.; Coustry, F.; Posey, K.L. Joint Degeneration in a Mouse Model of Pseudoachondroplasia: ER Stress, Inflammation, and Block of Autophagy. Int. J. Mol. Sci. 2021, 22, 9239. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Mortier, G.R.; Cole, W.G.; King, L.M.; Golik, S.S.; Bonaventure, J.; Nuytinck, L.; De Paepe, A.; Leroy, J.G.; Biesecker, L.; et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am. J. Hum. Genet. 1998, 62, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Pirog-Garcia, K.A.; Meadows, R.S.; Knowles, L.; Heinegard, D.; Thornton, D.J.; Kadler, K.E.; Boot-Handford, R.P.; Briggs, M.D. Reduced cell proliferation and increased apoptosis are significant pathological mechanisms in a murine model of mild pseudoachondroplasia resulting from a mutation in the C-terminal domain of COMP. Hum. Mol. Genet. 2007, 16, 2072–2088. [Google Scholar] [CrossRef]
- Jackson, G.C.; Mittaz-Crettol, L.; Taylor, J.A.; Mortier, G.R.; Spranger, J.; Zabel, B.; Le Merrer, M.; Cormier-Daire, V.; Hall, C.M.; Offiah, A.; et al. Pseudoachondroplasia and multiple epiphyseal dysplasia: A 7-year comprehensive analysis of the known disease genes identify novel and recurrent mutations and provides an accurate assessment of their relative contribution. Hum. Mutat. 2012, 33, 144–157. [Google Scholar] [CrossRef]
- Delot, E.; King, L.M.; Briggs, M.D.; Wilcox, W.R.; Cohn, D.H. Trinucleotide expansion mutations in the cartilage oligomeric matrix protein (COMP) gene. Hum. Mol. Genet. 1999, 8, 123–128. [Google Scholar] [CrossRef]
- Runhaar, J.; Kloppenburg, M.; Boers, M.; Bijlsma, J.W.J.; Bierma-Zeinstra, S.M.A.; CREDO expert group. Towards developing diagnostic criteria for early knee osteoarthritis: Data from the CHECK study. Rheumatology 2021, 60, 2448–2455. [Google Scholar] [CrossRef]
- Hao, H.Q.; Zhang, J.F.; He, Q.Q.; Wang, Z. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2019, 27, 726–736. [Google Scholar] [CrossRef]
- Riegger, J.; Rehm, M.; Buchele, G.; Brenner, H.; Gunther, K.P.; Rothenbacher, D.; Brenner, R.E. Serum Cartilage Oligomeric Matrix Protein in Late-Stage Osteoarthritis: Association with Clinical Features, Renal Function, and Cardiovascular Biomarkers. J. Clin. Med. 2020, 9, 268. [Google Scholar] [CrossRef]
- Henrotin, Y. Osteoarthritis in year 2021: Biochemical markers. Osteoarthr. Cartil. 2022, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Saxne, T.; Heinegard, D. Cartilage oligomeric matrix protein: A novel marker of cartilage turnover detectable in synovial fluid and blood. Br. J. Rheumatol. 1992, 31, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Manki, A.; Inoue, T.; Oda, M.; Tanaka, H.; Morishima, T. Clinical significance of decreased serum concentration of cartilage oligomeric matrix protein in systemic juvenile idiopathic arthritis. J. Rheumatol. 2006, 33, 996–1000. [Google Scholar] [PubMed]
- Recklies, A.D.; Baillargeon, L.; White, C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998, 41, 997–1006. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Saxne, T.; Heinegard, D.K. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann. Rheum. Dis. 1994, 53, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Akinmade, A.; Oginni, L.M.; Adegbehingbe, O.O.; Okunlola, A.I.; Jeje, O.A.; Adeyeye, A.I. Serum cartilage oligomeric matrix protein as a biomarker for predicting development and progression of knee osteoarthritis. Int. Orthop. 2021, 45, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hashimoto, Y.; Orita, K.; Nishino, K.; Kiinoshita, T.; Nakamura, H. Serum Cartilage Oligomeric Matrix Protein Detects Early Osteoarthritis in Patients With Anterior Cruciate Ligament Deficiency. Arthroscopy 2022, 38, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Algergawy, S.A.; Abd El-Sabour, M.; Osman, A.S.; Emam, S.M.; Elham, N. Early diagnostic and prognostic values of anti-cyclic citrullinated peptide antibody and cartilage oligomeric matrix protein in rheumatoid arthritis. Egypt J. Immunol. 2013, 20, 11–20. [Google Scholar]
- Liu, F.; Wang, X.; Zhang, X.; Ren, C.; Xin, J. Role of Serum cartilage oligomeric matrix protein (COMP) in the diagnosis of rheumatoid arthritis (RA): A case-control study. J. Int. Med. Res. 2016, 44, 940–949. [Google Scholar] [CrossRef]
- Saghafi, M.; Khodashahi, M.; Saadati, N.; Azarian, A.; Rezaieyazdi, Z.; Salehi, M.; Sahebari, M. Relationship between cartilage oligomeric matrix protein (COMP) and rheumatoid arthritis severity. Electron. Physician 2017, 9, 5940–5947. [Google Scholar] [CrossRef]
- Idriss, N.K.; Gamal, R.M.; Gaber, M.A.; El-Hakeim, E.H.; Hammam, N.; Ghandour, A.M.; Abdelaziz, M.M.; Goma, S.H. Joint remodeling outcome of serum levels of Dickkopf-1 (DKK1), cartilage oligomeric matrix protein (COMP), and C-telopeptide of type II collagen (CTXII) in rheumatoid arthritis. Cent. Eur. J. Immunol. 2020, 45, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.L.; Svensson, B.; Petersson, I.F.; Hafstrom, I.; Albertsson, K.; Forslind, K.; Heinegard, D.; Saxne, T. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2013, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, S.; Hansson, A.S.; Olsson, H.; Heinegard, D.; Holmdahl, R. Cartilage oligomeric matrix protein (COMP)-induced arthritis in rats. Clin. Exp. Immunol. 1998, 114, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, S.; Nandakumar, K.S.; Backlund, J.; Holmberg, J.; Hultqvist, M.; Vestberg, M.; Holmdahl, R. Cartilage oligomeric matrix protein induction of chronic arthritis in mice. Arthritis Rheum. 2008, 58, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Nandakumar, K.S.; Pramhed, A.; Aspberg, A.; Mattsson, R.; Holmdahl, R. Cartilage oligomeric matrix protein specific antibodies are pathogenic. Arthritis Res. Ther. 2012, 14, R191. [Google Scholar] [CrossRef]
- Li, Y.; Tong, D.; Liang, P.; Lonnblom, E.; Viljanen, J.; Xu, B.; Nandakumar, K.S.; Holmdahl, R. Cartilage-binding antibodies initiate joint inflammation and promote chronic erosive arthritis. Arthritis Res. Ther. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Souto-Carneiro, M.M.; Burkhardt, H.; Muller, E.C.; Hermann, R.; Otto, A.; Kraetsch, H.G.; Sack, U.; Konig, A.; Heinegard, D.; Muller-Hermelink, H.K.; et al. Human monoclonal rheumatoid synovial B lymphocyte hybridoma with a new disease-related specificity for cartilage oligomeric matrix protein. J. Immunol. 2001, 166, 4202–4208. [Google Scholar] [CrossRef]
- Ge, C.; Tong, D.; Lonnblom, E.; Liang, B.; Cai, W.; Fahlquist-Hagert, C.; Li, T.; Kastbom, A.; Gjertsson, I.; Dobritzsch, D.; et al. Antibodies to Cartilage Oligomeric Matrix Protein Are Pathogenic in Mice and May Be Clinically Relevant in Rheumatoid Arthritis. Arthritis Rheumatol. 2022, 74, 961–971. [Google Scholar] [CrossRef]
- Qi, D.D.; Liu, Z.H.; Wu, D.S.; Huang, Y.F. A Study on COMP and CTX-II as Molecular Markers for the Diagnosis of Intervertebral Disc Degeneration. Biomed. Res. Int. 2021, 2021, 3371091. [Google Scholar] [CrossRef]
- Ganu, V.; Goldberg, R.; Peppard, J.; Rediske, J.; Melton, R.; Hu, S.I.; Wang, W.; Duvander, C.; Heinegard, D. Inhibition of interleukin-1alpha-induced cartilage oligomeric matrix protein degradation in bovine articular cartilage by matrix metalloproteinase inhibitors: Potential role for matrix metalloproteinases in the generation of cartilage oligomeric matrix protein fragments in arthritic synovial fluid. Arthritis Rheum. 1998, 41, 2143–2151. [Google Scholar] [CrossRef]
- Giannoni, P.; Siegrist, M.; Hunziker, E.B.; Wong, M. The mechanosensitivity of cartilage oligomeric matrix protein (COMP). Biorheology 2003, 40, 101–109. [Google Scholar] [PubMed]
- Wong, M.; Siegrist, M.; Cao, X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999, 18, 391–399. [Google Scholar] [CrossRef]
- Speichert, S.; Molotkov, N.; El Bagdadi, K.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Role of Norepinephrine in IL-1beta-Induced Chondrocyte Dedifferentiation under Physioxia. Int. J. Mol. Sci. 2019, 20, 1212. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin beta1-SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhu, Y. Fibronectin fragment activation of ERK increasing integrin alpha(5) and beta(1) subunit expression to degenerate nucleus pulposus cells. J. Orthop. Res. 2011, 29, 556–561. [Google Scholar] [CrossRef]
- Prasadam, I.; Friis, T.; Shi, W.; van Gennip, S.; Crawford, R.; Xiao, Y. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone 2010, 46, 226–235. [Google Scholar] [CrossRef]
- Zhao, Y.; Urbonaviciute, V.; Xu, B.; Cai, W.; Sener, Z.; Ge, C.; Holmdahl, R. Cartilage Oligomeric Matrix Protein Induced Arthritis-A New Model for Rheumatoid Arthritis in the C57BL/6 Mouse. Front. Immunol. 2021, 12, 631249. [Google Scholar] [CrossRef]
- Ishii, Y.; Thomas, A.O.; Guo, X.E.; Hung, C.T.; Chen, F.H. Localization and distribution of cartilage oligomeric matrix protein in the rat intervertebral disc. Spine 2006, 31, 1539–1546. [Google Scholar] [CrossRef]
- Gorth, D.J.; Ottone, O.K.; Shapiro, I.M.; Risbud, M.V. Differential Effect of Long-Term Systemic Exposure of TNFalpha on Health of the Annulus Fibrosus and Nucleus Pulposus of the Intervertebral Disc. J. Bone Miner. Res. 2020, 35, 725–737. [Google Scholar] [CrossRef]
- Yee, A.; Lam, M.P.; Tam, V.; Chan, W.C.; Chu, I.K.; Cheah, K.S.; Cheung, K.M.; Chan, D. Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthr. Cartil. 2016, 24, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liang, X.; Wang, X.; Zhang, L.; Cheng, S.; Guo, X.; Zhang, F.; Wen, Y. The molecular mechanism study of COMP involved in the articular cartilage damage of Kashin-Beck disease. Bone Jt. Res. 2020, 9, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Kleeff, J.; Guo, J.; Gazdhar, A.; Liao, Q.; Di Cesare, P.E.; Buchler, M.W.; Friess, H. Cartilage oligomeric matrix protein expression in hepatocellular carcinoma and the cirrhotic liver. J. Gastroenterol. Hepatol. 2004, 19, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Englund, E.; Bartoschek, M.; Reitsma, B.; Jacobsson, L.; Escudero-Esparza, A.; Orimo, A.; Leandersson, K.; Hagerling, C.; Aspberg, A.; Storm, P.; et al. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene 2016, 35, 5585–5596. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.L.; Gatselis, N.K.; Shums, Z.; Liaskos, C.; Bogdanos, D.P.; Koukoulis, G.K.; Dalekos, G.N. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Piratvisuth, T.; Tanwandee, T.; Thongsawat, S.; Sukeepaisarnjaroen, W.; Esteban, J.I.; Bes, M.; Kohler, B.; He, Y.; Swiatek-de Lange, M.; Morgenstern, D.; et al. Multimarker Panels for Detection of Early Stage Hepatocellular Carcinoma: A Prospective, Multicenter, Case-Control Study. Hepatol. Commun. 2022, 6, 679–691. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Wang, Y.; Sun, L.; Liu, Z.; Wang, L.; Song, T.; Yao, Y.; Liu, Q.; Tu, K. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J. Exp. Clin. Cancer Res. 2018, 37, 231. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, Q.; Fang, S.; Liu, M.; Li, Y.; Yuan, Y.F.; Li, Y.; Zhu, Y.; Qi, J.; Yang, X.; et al. Calcium-binding protein 39 promotes hepatocellular carcinoma growth and metastasis by activating extracellular signal-regulated kinase signaling pathway. Hepatology 2017, 66, 1529–1545. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, L.; Yao, B.; Chen, T.; Li, Q.; Liu, Z.; Liu, R.; Niu, Y.; Song, T.; et al. Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J. Exp. Clin. Cancer Res. 2019, 38, 170. [Google Scholar] [CrossRef]
- Liu, T.T.; Liu, X.S.; Zhang, M.; Liu, X.N.; Zhu, F.X.; Zhu, F.M.; Ouyang, S.W.; Li, S.B.; Song, C.L.; Sun, H.M.; et al. Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the Akt pathway. J. Cancer Res. Clin. Oncol. 2018, 144, 1049–1063. [Google Scholar] [CrossRef]
- Wusterbarth, E.; Chen, Y.; Jecius, H.; Krall, E.; Runyan, R.B.; Pandey, R.; Nfonsam, V. Cartilage Oligomeric Matrix Protein, COMP may be a Better Prognostic Marker Than CEACAM5 and Correlates With Colon Cancer Molecular Subtypes, Tumor Aggressiveness and Overall Survival. J. Surg. Res. 2022, 270, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, K.S.; Darlix, A.; Jacot, W.; Blom, A.M. High Levels of Cartilage Oligomeric Matrix Protein in the Serum of Breast Cancer Patients Can Serve as an Independent Prognostic Marker. Front. Oncol. 2019, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal. 2021, 14, eabj2807. [Google Scholar] [CrossRef]
- Englund, E.; Canesin, G.; Papadakos, K.S.; Vishnu, N.; Persson, E.; Reitsma, B.; Anand, A.; Jacobsson, L.; Helczynski, L.; Mulder, H.; et al. Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget 2017, 8, 98298–98311. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, K.S.; Bartoschek, M.; Rodriguez, C.; Gialeli, C.; Jin, S.B.; Lendahl, U.; Pietras, K.; Blom, A.M. Cartilage Oligomeric Matrix Protein initiates cancer stem cells through activation of Jagged1-Notch3 signaling. Matrix Biol. 2019, 81, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, B.; Liu, M.; Qiao, H.; Zhang, S.; Qiu, J.; Ying, X. Long non-coding RNA SNHG25 promotes epithelial ovarian cancer progression by up-regulating COMP. J. Cancer 2021, 12, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Xiang, H.; Wu, X.; Tang, Q.; Ma, X.; Zhang, L. ADAMTS7 degrades Comp to fuel BMP2-dependent osteogenic differentiation and ameliorate oncogenic potential in osteosarcomas. FEBS Open Bio. 2020, 10, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, X.; Ren, Y.; Yin, Z.; Zhou, B. Coexisting EGFR and TP53 Mutations in Lung Adenocarcinoma Patients Are Associated With COMP and ITGB8 Upregulation and Poor Prognosis. Front. Mol. Biosci. 2020, 7, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Lv, C.; Han, J.; Hao, M.; Li, J.; Qiao, H. Cartilage oligomeric matrix protein affects the biological behavior of papillary thyroid carcinoma cells by activating the PI3K/AKT/Bcl-2 pathway. J. Cancer 2021, 12, 1623–1633. [Google Scholar] [CrossRef]
- Han, J.; Chen, M.; Wang, Y.; Gong, B.; Zhuang, T.; Liang, L.; Qiao, H. Identification of Biomarkers Based on Differentially Expressed Genes in Papillary Thyroid Carcinoma. Sci Rep. 2018, 8, 9912. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, S.; Jing, J. Identifying Diagnostic and Prognostic Biomarkers and Candidate Therapeutic Drugs of Gastric Cancer Based on Transcriptomics and Single-Cell Sequencing. Pathol. Oncol. Res. 2021, 27, 1609955. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jia, Q.; Feng, X.; Chen, H.; Wang, L.; Ni, X.; Kong, W. Hypoxia decrease expression of cartilage oligomeric matrix protein to promote phenotype switching of pulmonary arterial smooth muscle cells. Int. J. Biochem. Cell Biol. 2017, 91, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Alruwaili, N.; Hu, B.; Kelly, M.R.; Zhang, B.; Sun, D.; Wolin, M.S. Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia. Am. J. Physiol Lung Cell Mol. Physiol. 2019, 317, L569–L577. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Cao, Y.; Li, L.; Chen, W.; Chen, X. Upregulation of ADAMTS7 and downregulation of COMP are associated with aortic aneurysm. Mol. Med. Rep. 2017, 16, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Y.; Gao, C.; Jia, Y.; Huang, Y.; Liu, L.; Wang, X.; Wang, W.; Kong, W. Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence. Biochem. Biophys. Res. Commun. 2016, 478, 1006–1013. [Google Scholar] [CrossRef]
- Fu, Y.; Kong, W. Cartilage Oligomeric Matrix Protein: Matricellular and Matricrine Signaling in Cardiovascular Homeostasis and Disease. Curr. Vasc. Pharmacol. 2017, 15, 186–196. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, C.; Liang, Y.; Wang, M.; Huang, Y.; Ma, W.; Li, T.; Jia, Y.; Yu, F.; Zhu, W.; et al. Shift of Macrophage Phenotype Due to Cartilage Oligomeric Matrix Protein Deficiency Drives Atherosclerotic Calcification. Circ. Res. 2016, 119, 261–276. [Google Scholar] [CrossRef]
- Lv, H.; Wang, H.; Quan, M.; Zhang, C.; Fu, Y.; Zhang, L.; Lin, C.; Liu, X.; Yi, X.; Chen, J.; et al. Cartilage oligomeric matrix protein fine-tunes disturbed flow-induced endothelial activation and atherogenesis. Matrix Biol. 2021, 95, 32–51. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Y.; Wang, L.; Liu, B.; Tian, Q.; Liu, C.J.; Zhang, T.; Xu, Q.; Zhu, Y.; Ake, O.; et al. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ. Res. 2011, 108, 917–928. [Google Scholar] [CrossRef]
- Hultman, K.; Edsfeldt, A.; Bjorkbacka, H.; Duner, P.; Sundius, L.; Nitulescu, M.; Persson, A.; Boyle, J.J.; Nilsson, J.; Hultgardh-Nilsson, A.; et al. Cartilage Oligomeric Matrix Protein Associates With a Vulnerable Plaque Phenotype in Human Atherosclerotic Plaques. Stroke 2019, 50, 3289–3292. [Google Scholar] [CrossRef]
- Sandstedt, J.; Vargmar, K.; Bjorkman, K.; Ruetschi, U.; Bergstrom, G.; Hulten, L.M.; Skioldebrand, E. COMP (Cartilage Oligomeric Matrix Protein) Neoepitope: A Novel Biomarker to Identify Symptomatic Carotid Stenosis. Arter. Thromb. Vasc. Biol. 2021, 41, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Zachou, K.; Gabeta, S.; Shums, Z.; Gatselis, N.K.; Koukoulis, G.K.; Norman, G.L.; Dalekos, G.N. COMP serum levels: A new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. Eur. J. Intern. Med. 2017, 38, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Farina, G.; Lemaire, R.; Korn, J.H.; Widom, R.L. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006, 25, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Shono, F.; Nakajima, T.; Hosokawa, K.; Itami, S. Identification and characterization of cartilage oligomeric matrix protein as a novel pathogenic factor in keloids. Am. J. Pathol. 2011, 179, 1951–1960. [Google Scholar] [CrossRef]
- Agarwal, P.; Schulz, J.N.; Blumbach, K.; Andreasson, K.; Heinegard, D.; Paulsson, M.; Mauch, C.; Eming, S.A.; Eckes, B.; Krieg, T. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol. 2013, 32, 325–331. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar] [CrossRef]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Farina, G.; Lemaire, R.; Pancari, P.; Bayle, J.; Widom, R.L.; Lafyatis, R. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann. Rheum. Dis. 2009, 68, 435–441. [Google Scholar] [CrossRef]
- Magdaleno, F.; Arriazu, E.; Ruiz de Galarreta, M.; Chen, Y.; Ge, X.; Conde de la Rosa, L.; Nieto, N. Cartilage oligomeric matrix protein participates in the pathogenesis of liver fibrosis. J. Hepatol. 2016, 65, 963–971. [Google Scholar] [CrossRef]
- Vuga, L.J.; Milosevic, J.; Pandit, K.; Ben-Yehudah, A.; Chu, Y.; Richards, T.; Sciurba, J.; Myerburg, M.; Zhang, Y.; Parwani, A.V.; et al. Cartilage oligomeric matrix protein in idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e83120. [Google Scholar] [CrossRef]
- Bartosinska, J.; Michalak-Stoma, A.; Juszkiewicz-Borowiec, M.; Kowal, M.; Chodorowska, G. The Assessment of Selected Bone and Cartilage Biomarkers in Psoriatic Patients from Poland. Mediat. Inflamm. 2015, 2015, 194535. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Cook, R.J.; Edwin, J.; Shen, H.; Pellett, F.J.; Shanmugarajah, S.; Rosen, C.F.; Gladman, D.D. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology 2010, 49, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Abji, F.; Perruccio, A.V.; Gandhi, R.; Li, S.; Cook, R.J.; Gladman, D.D. Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis. Ann. Rheum. Dis. 2019, 78, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Bozo, R.; Szel, E.; Danis, J.; Guban, B.; Bata-Csorgo, Z.; Szabo, K.; Kemeny, L.; Groma, G. Cartilage Oligomeric Matrix Protein Negatively Influences Keratinocyte Proliferation via alpha5beta1-Integrin: Potential Relevance of Altered Cartilage Oligomeric Matrix Protein Expression in Psoriasis. J. Investig. Dermatol. 2020, 140, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Denton, N.; Pinnick, K.E.; Karpe, F. Cartilage oligomeric matrix protein is differentially expressed in human subcutaneous adipose tissue and regulates adipogenesis. Mol. Metab. 2018, 16, 172–179. [Google Scholar] [CrossRef]
- Jansa, V.; Klancic, T.; Pusic, M.; Klein, M.; Vrtacnik Bokal, E.; Ban Frangez, H.; Rizner, T.L. Proteomic analysis of peritoneal fluid identified COMP and TGFBI as new candidate biomarkers for endometriosis. Sci. Rep. 2021, 11, 20870. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; He, J.; Zhang, R.; Cao, Y.; Liu, X. A novel mutation in exon 11 of COMP gene in a Chinese family with pseudoachondroplasia. Genes Dis. 2019, 6, 47–55. [Google Scholar] [CrossRef]
- Hecht, J.T.; Coustry, F.; Veerisetty, A.C.; Hossain, M.G.; Posey, K.L. Resveratrol Reduces COMPopathy in Mice Through Activation of Autophagy. JBMR Plus 2021, 5, e10456. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23169253
Cui J, Zhang J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. International Journal of Molecular Sciences. 2022; 23(16):9253. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23169253
Chicago/Turabian StyleCui, Jiarui, and Jiaming Zhang. 2022. "Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities" International Journal of Molecular Sciences 23, no. 16: 9253. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23169253