Obstructive Sleep Apnea Syndrome In Vitro Model: Controlled Intermittent Hypoxia Stimulation of Human Stem Cells-Derived Cardiomyocytes
Abstract
:1. Introduction
2. Results
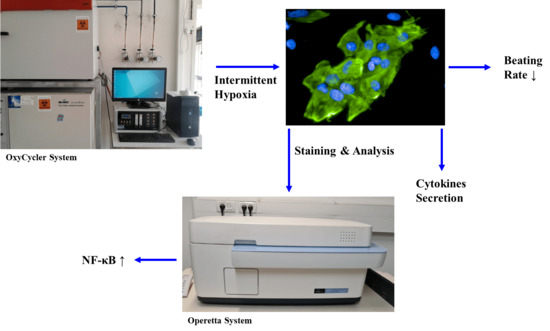
2.1. Hif-1α Expression Is Increased in hESC-CMs following IH
2.2. Decrease in hESC-CMs Beating Rate following IH
2.3. Increased NF-κB Activation following IH in hESC-CMs
2.4. Detection of Cytokines following IH in hESC-CMs
3. Discussion
4. Materials and Methods
4.1. Antibodies
4.2. Cardiomyocytes (CMs) Differentiation
4.3. Hypoxia Induction by Using the “OxyCycler System”
4.4. Beating Rate Measurement
4.5. Operetta High-Content Imaging System—PerkinElmer
4.6. Determination of Specific Proteins in Supernatants
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. The Association of Obstructive Sleep Apnea and Hypertension. Cureus 2019, 11, e4858. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Parish, J.M.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease. 2004. Available online: www.mayo.edu/proceedings (accessed on 29 July 2020).
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent Hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, W.T.; Bonsignore, M.R. Sleep apnoea as an independent risk factor for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur. Respir. J. 2007, 29, 156–178. [Google Scholar] [CrossRef]
- Gupta, N.; Ashraf, M.Z. Hypoxia Signaling in Cardiovascular Diseases. In Hypoxia and Anoxia; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Belaidi, E.; Thomas, A.; Bourdier, G.; Moulin, S.; Lemarié, E.; Levy, P.; Pépin, J.-L.; Korichneva, I.; Godin-Ribuot, D.; Arnaud, C. Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: Its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int. J. Cardiol. 2016, 210, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 24, 884–889. [Google Scholar] [CrossRef]
- Wei, Q.; Bian, Y.; Yu, F.; Zhang, Q.; Zhang, G.; Li, Y.; Song, S.; Ren, X.; Tong, J. Chronic intermittent hypoxia induces cardiac inflammation anddysfunction in a rat obstructive sleep apnea model. J. Biomed. Res. 2016, 30, 490–495. [Google Scholar] [CrossRef]
- Williams, A.; Scharf, S.M. Obstructive sleep apnea, cardiovascular disease, and inflammation—Is NF-κB the key? Sleep Breath. 2007, 11, 69–76. [Google Scholar] [CrossRef]
- Israel, L.P.; Benharoch, D.; Gopas, J.; Goldbart, A.D. A Pro-Inflammatory Role for Nuclear Factor Kappa B in Childhood Obstructive Sleep Apnea Syndrome. Sleep 2013, 36, 1947–1955. [Google Scholar] [CrossRef] [Green Version]
- Goldbart, A.D.; Gannot, M.; Haddad, H.; Gopas, J. Nuclear factor kappa B activation in cardiomyocytes by serum of children with obstructive sleep apnea syndrome. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Haddad, H.; Etzion, S.; Rabinski, T.; Ofir, R.; Regev, D.; Etzion, Y.; Gopas, J.; Goldbart, A. The Effect of Sera from Children with Obstructive Sleep Apnea Syndrome (OSAS) on Human Cardiomyocytes Differentiated from Human Embryonic Stem Cells. Int. J. Mol. Sci. 2021, 22, 11418. [Google Scholar] [CrossRef] [PubMed]
- Baguet, J.-P.; Barone-Rochette, G.; Tamisier, R.; Lévy, P.; Pépin, J.L. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat. Rev. Cardiol. 2012, 9, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, B.Y.-M.; Liu, Y.; Liu, Y. Meta-Analysis of the Effect of Obstructive Sleep Apnea on Cardiovascular Events After Percutaneous Coronary Intervention. Am. J. Cardiol. 2017, 120, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Reynor, A.; McArdle, N.; Shenoy, B.; Dhaliwal, S.S.; Rea, S.C.; Walsh, J.; Eastwood, P.R.; Maddison, K.; Hillman, D.R.; Ling, I.; et al. Continuous positive airway pressure and adverse cardiovascular events in obstructive sleep apnea: Are participants of randomized trials representative of sleep clinic patients? Sleep 2021, 45, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Szmyd, B.; Szemraj, J.; Stawski, R.; Sochal, M.; Białasiewicz, P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J. Clin. Sleep Med. 2020, 16, 1761. [Google Scholar] [CrossRef] [PubMed]
- Stroka, D.M.; Burkhardt, T.; Desbaillets, I.; Wenger, R.H.; Neil, D.A.H.; Bauer, C.; Gassmann, M.; Candinas, D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001, 15, 2445–2453. [Google Scholar] [CrossRef]
- Murphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pépin, J.L.; Roche, H.; Arnaud, C.; Ryan, S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Zhu, B.; Luo, A.-L.; Yang, L.; Yang, C. The Role of Cardiokines in Heart Diseases: Beneficial or Detrimental? BioMed Res. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testelmans, D.; Tamisier, R.; Barone-Rochette, G.; Baguet, J.-P.; Roux-Lombard, P.; Pépin, J.-L.; Lévy, P. Profile of circulating cytokines: Impact of OSA, obesity and acute cardiovascular events. Cytokine 2013, 62, 210–216. [Google Scholar] [CrossRef]
- Taniguchi, K.; Shimouchi, A.; Jinno, N.; Okumura, N.; Seiyama, A. Parasympathetic Nervous Activity Associated with Discoordination between Physical Acceleration and Heart Rate Variability in Patients with Sleep Apnea; Springer: Cham, Switzerland, 2021; Volume 1269, pp. 229–234. [Google Scholar] [CrossRef]
- Leung, R.S. Sleep-Disordered Breathing: Autonomic Mechanisms and Arrhythmias. Prog. Cardiovasc. Dis. 2009, 51, 324–338. [Google Scholar] [CrossRef]
- Kitajima, I.; Soejima, Y.; Takasaki, I.; Beppu, H.; Tokioka, T.; Maruyama, I. Ceramide-induced nuclear translocation of NF-κB is a potential mediator of the apoptotic response to TNF-α in murine clonal osteoblasts. Bone 1996, 19, 263–270. [Google Scholar] [CrossRef]
- Rivera-Serrano, E.E.; Sherry, B. NF-κB activation is cell type-specific in the heart. Virology 2016, 502, 133–143. [Google Scholar] [CrossRef]
- Kim, Y.S. BAY 11-7082, a Nuclear Factor-κB Inhibitor, Reduces Inflammation and Apoptosis in a Rat Cardiac Ischemia-Reperfusion Injury Model. Int. Heart J. 2010, 51, 348–353. [Google Scholar] [CrossRef]
- BDas, B.; Gupta, S.; Vasanji, A.; Xu, Z.; Misra, S.; Sen, S. Nuclear Co-translocation of Myotrophin and p65 Stimulates Myocyte Growth: Regulation by myotrophin hairpin loops. J. Biol. Chem. 2008, 283, 27947–27956. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of Elevated Nuclear Factor-κB–dependent Genes in Obstructive Sleep Apnea Syndrome. Am. J. Respir. Crit. Care Med. 2012, 174, 824–830. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective Activation of Inflammatory Pathways by Intermittent Hypoxia in Obstructive Sleep Apnea Syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, E.; García-Tovar, S.; Alfaro, E.; Jaureguizar, A.; Casitas, R.; Sánchez-Sánchez, B.; Zamarrón, E.; Fernández-Lahera, J.; López-Collazo, E.; Cubillos-Zapata, C.; et al. Inflammasome Activation: A Keystone of Proinflammatory Response in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Borker, P.V.; Patel, S.R. Monocyte Activation: The Link between Obstructive Sleep Apnea and Cardiovascular Disease? Am. J. Respir. Crit. Care Med. 2022, 205, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Tilstam, P.V.; Qi, D.; Leng, L.; Young, L.; Bucala, R. MIF family cytokines in cardiovascular diseases and prospects for precision-based therapeutics. Expert Opin. Ther. Targets 2017, 21, 671–683. [Google Scholar] [CrossRef]
- Wu, J.; Stefaniak, J.; Hafner, C.; Schramel, J.P.; Kaun, C.; Wojta, J.; Ullrich, R.; Tretter, V.E.; Markstaller, K.; Klein, K.U. Intermittent Hypoxia Causes Inflammation and Injury to Human Adult Cardiac Myocytes. Anesthesia Analg. 2016, 122, 373–380. [Google Scholar] [CrossRef]
- Edwards, K.M.; Tomfohr, B.L.M.; Mills, P.J.; Bosch, J.A.; Ancoli-Lsrael, S.; Loredo, J.S.; Dimsdale, J. Macrophage Migratory Inhibitory Factor (MIF) May Be a Key Factor in Inflammation in Obstructive Sleep Apnea. Sleep 2011, 34, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Dayawansa, N.H.; Gao, X.-M.; White, D.A.; Dart, A.; Du, X.-J. Role of MIF in myocardial ischaemia and infarction: Insight from recent clinical and experimental findings. Clin. Sci. 2014, 127, 149–161. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishihira, J.; Shimpo, M.; Mizue, Y.; Ueno, S.; Mano, H.; Kobayashi, E.; Ikeda, U.; Shimada, K. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc. Res. 2001, 52, 438–445. [Google Scholar] [CrossRef]
- Jian, Z.; Li, J.-B.; Ma, R.-Y.; Chen, L.; Zhong, Q.-J.; Wang, X.-F.; Wang, W.; Hong, Y.; Xiao, Y.-B. Increase of macrophage migration inhibitory factor (MIF) expression in cardiomyocytes during chronic hypoxia. Clin. Chim. Acta 2009, 405, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Kvakan, H.; Kaun, C.; Zorn, G.; Speidl, W.; Pfaffenberger, S.; Maurer, G.; Huber, K.; Wojta, J. The gp130 ligand oncostatin M regulates tissue inhibitor of metalloproteinases-1 through ERK1/2 and p38 in human adult cardiac myocytes and in human adult cardiac fibroblasts: A possible role for the gp130/gp130 ligand system in the modulation of extracellular matrix degradation in the human heart. J. Mol. Cell. Cardiol. 2005, 39, 545–551. [Google Scholar] [CrossRef]
- Xu, X.-M.; Yao, D.; Cai, X.-D.; Ding, C.; Lin, Q.-D.; Wang, L.-X.; Huang, X.-Y. Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats. Sleep Breath. 2014, 19, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Kimura, D. Hypoxia Enhances the Expression of Plasminogen Activator Inhibitor-1 in Human Lung Cancer Cells, EBC-1. Tohoku J. Exp. Med. 2022, 196, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricke-Hoch, M.; Hoes, M.F.; Pfeffer, T.J.; Schlothauer, S.; Nonhoff, J.; Haidari, S.; Bomer, N.; Scherr, M.; Stapel, B.; Stelling, E.; et al. In peripartum cardiomyopathy plasminogen activator inhibitor-1 is a potential new biomarker with controversial roles. Cardiovasc. Res. 2019, 116, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Tarbit, E.; Singh, I.; Peart, J.N.; Rose’Meyer, R.B. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Hear. Fail. Rev. 2018, 24, 1. [Google Scholar] [CrossRef]
- Hohensinner, P.; Kaun, C.; Rychli, K.; Cohen, E.B.-T.; Kastl, S.P.; Demyanets, S.; Pfaffenberger, S.; Speidl, W.; Rega, G.; Ullrich, R.; et al. Monocyte chemoattractant protein (MCP-1) is expressed in human cardiac cells and is differentially regulated by inflammatory mediators and hypoxia. FEBS Lett. 2006, 580, 3532–3538. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.-P.; Chen, N.-H.; Lin, S.-W.; Hu, H.-C.; Kao, K.-C.; Li, L.-F.; Yang, C.-T.; Pang, J.-H.S. Monocytic C-C chemokine receptor 5 expression increases in in vitro intermittent hypoxia condition and in severe obstructive sleep apnea patients. Sleep Breath. 2019, 23, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Yin, R.; Wang, S.-N.; Ying, R. A Review of CXCL1 in Cardiac Fibrosis. Front. Cardiovasc. Med. 2021, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.-L.; Lin, Q.-Y.; Liu, Y.; Guan, X.-M.; Ma, X.-L.; Cao, H.-J.; Liu, Y.; Bai, J.; Xia, Y.-L.; et al. CXCL1–CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur. Hear. J. 2018, 39, 1818–1831. [Google Scholar] [CrossRef]
- Choi, S.-C.; Seo, H.-R.; Cui, L.-H.; Song, M.-H.; Noh, J.-M.; Kim, K.-S.; Choi, J.-H.; Kim, J.-H.; Park, C.-Y.; Joo, H.J.; et al. Modeling Hypoxic Stress In Vitro Using Human Embryonic Stem Cells Derived Cardiomyocytes Matured by FGF4 and Ascorbic Acid Treatment. Cells 2021, 10, 2741. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Induction and Testing of Hypoxia in Cell Culture. J. Vis. Exp. 2011, 2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regev, D.; Etzion, S.; Haddad, H.; Gopas, J.; Goldbart, A. Obstructive Sleep Apnea Syndrome In Vitro Model: Controlled Intermittent Hypoxia Stimulation of Human Stem Cells-Derived Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 10272. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810272
Regev D, Etzion S, Haddad H, Gopas J, Goldbart A. Obstructive Sleep Apnea Syndrome In Vitro Model: Controlled Intermittent Hypoxia Stimulation of Human Stem Cells-Derived Cardiomyocytes. International Journal of Molecular Sciences. 2022; 23(18):10272. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810272
Chicago/Turabian StyleRegev, Danielle, Sharon Etzion, Hen Haddad, Jacob Gopas, and Aviv Goldbart. 2022. "Obstructive Sleep Apnea Syndrome In Vitro Model: Controlled Intermittent Hypoxia Stimulation of Human Stem Cells-Derived Cardiomyocytes" International Journal of Molecular Sciences 23, no. 18: 10272. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810272