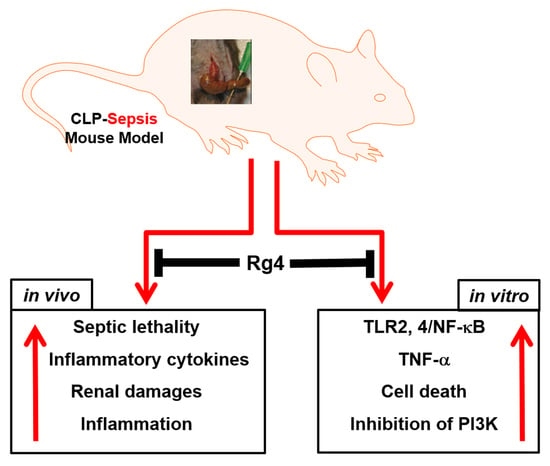
Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis
Abstract
:1. Introduction
2. Results
2.1. Protective Effects of Rg4 on CLP-Induced Sepsis
2.2. Inhibitory Effects of Rg4 on Inflammatory Cytokine Levels and Renal Inflammation in CLP-Operated Mice
2.3. Inhibitory Effects of Rg4 on TLR and NF-κB Expression
2.4. Effects of Rg4 on Cell Survival In Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Animals and the CLP Procedure
4.3. Experimental Design
4.4. Hematoxylin and Eosin (H&E) Staining and Histopathological Examination
4.5. Biochemical Measurement
4.6. Cell Viability Assay
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dong, H.; Bai, L.P.; Wong, V.K.; Zhou, H.; Wang, J.R.; Liu, Y.; Jiang, Z.H.; Liu, L. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules 2011, 16, 10619–10630. [Google Scholar] [CrossRef]
- Wu, J.Y.; Gardner, B.H.; Murphy, C.I.; Seals, J.R.; Kensil, C.R.; Recchia, J.; Beltz, G.A.; Newman, G.W.; Newman, M.J. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J. Immunol. 1992, 148, 1519–1525. [Google Scholar] [PubMed]
- Nag, S.A.; Qin, J.J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R.W. Ginsenosides as anticancer agents: In vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological Activities and Chemistry of Saponins from Panax ginseng C. A. Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar] [CrossRef]
- Kang, O.H.; Shon, M.Y.; Kong, R.; Seo, Y.S.; Zhou, T.; Kim, D.Y.; Kim, Y.S.; Kwon, D.Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement. Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.S.; Kim, C.E.; Shin, M.S.; Seo, C.S.; Shin, H.K.; Hwang, G.S.; An, J.M.; Kim, S.N.; Kang, K.S. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2018, 42, 524–531. [Google Scholar] [CrossRef]
- Saba, E.; Lee, Y.Y.; Kim, M.; Kim, S.H.; Hong, S.B.; Rhee, M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J. Ginseng Res. 2018, 42, 577–584. [Google Scholar] [CrossRef]
- Sun, B.S.; Gu, L.J.; Fang, Z.M.; Wang, C.Y.; Wang, Z.; Lee, M.R.; Li, Z.; Li, J.J.; Sung, C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef]
- Cho, J.H.; Chun, H.Y.; Lee, J.S.; Lee, J.H.; Cheong, K.J.; Jung, Y.S.; Woo, T.G.; Yoon, M.H.; Oh, A.Y.; Kang, S.M.; et al. Prevention effect of rare ginsenosides against stress-hormone induced MTOC amplification. Oncotarget 2016, 7, 35144–35158. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, H.; Shehzad, O.; Kim, Y.S.; Kim, H.P. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur. J. Pharmacol. 2014, 724, 145–151. [Google Scholar] [CrossRef]
- Chen, B.; Shen, Y.P.; Zhang, D.F.; Cheng, J.; Jia, X.B. The apoptosis-inducing effect of ginsenoside F4 from steamed notoginseng on human lymphocytoma JK cells. Nat. Prod. Res. 2013, 27, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, G.E.; Song, G.Y.; Bae, J.S. Pulmonary protective functions of rare ginsenoside Rg4 on particulate matter-induced inflammatory responses. Biotechnol. Bioprocess Eng. 2019, 24, 445–453. [Google Scholar] [CrossRef]
- Bae, J.S. Role of high mobility group box 1 in inflammatory disease: Focus on sepsis. Arch. Pharm. Res. 2012, 35, 1511–1523. [Google Scholar] [CrossRef]
- Schefold, J.C.; Hasper, D.; Reinke, P.; Monneret, G.; Volk, H.D. Consider delayed immunosuppression into the concept of sepsis. Crit. Care Med. 2008, 36, 3118. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Tanaka, N.; Inaba, Y.; Gando, S.; Shiraishi, A.; Saitoh, D.; Otomo, Y.; Ikeda, H.; Ogura, H.; Kushimoto, S.; et al. Predictors of severe sepsis-related in-hospital mortality based on a multicenter cohort study: The Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis, and Trauma study. Medicine 2021, 100, e24844. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.; Lehmann, C. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int. J. Mol. Sci. 2019, 20, 4341. [Google Scholar] [CrossRef]
- Ziesmann, M.T.; Marshall, J.C. Multiple Organ Dysfunction: The Defining Syndrome of Sepsis. Surg. Infect. 2018, 19, 184–190. [Google Scholar] [CrossRef]
- Thachil, J.; Toh, C.H.; Levi, M.; Watson, H.G. The withdrawal of Activated Protein C from the use in patients with severe sepsis and DIC [Amendment to the BCSH guideline on disseminated intravascular coagulation]. Br. J. Haematol. 2012, 157, 493–494. [Google Scholar] [CrossRef]
- Rittirsch, D.; Hoesel, L.M.; Ward, P.A. The disconnect between animal models of sepsis and human sepsis. J. Leukoc. Biol. 2007, 81, 137–143. [Google Scholar] [CrossRef]
- Buras, J.A.; Holzmann, B.; Sitkovsky, M. Animal models of sepsis: Setting the stage. Nat. Rev. Drug Discov. 2005, 4, 854–865. [Google Scholar] [CrossRef]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, H.; Lee, T.; Park, E.K.; Bae, J.S. Inhibitory functions of maslinic acid, a natural triterpene, on HMGB1-mediated septic responses. Phytomedicine 2020, 69, 153200. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Bouchard, J.; Soroko, S.B.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M.; Himmelfarb, J.; Program to Improve Care in Acute Renal Disease Study Group. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011, 37, 241–248. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, J.H.; Kwon, H.M.; Lee, D.H.; Won, M.H.; Kwon, Y.G.; Kim, Y.M. Korean Red Ginseng protects endothelial cells from serum-deprived apoptosis by regulating Bcl-2 family protein dynamics and caspase S-nitrosylation. J. Ginseng Res. 2013, 37, 413–424. [Google Scholar] [CrossRef]
- Kim, Y.M.; Namkoong, S.; Yun, Y.G.; Hong, H.D.; Lee, Y.C.; Ha, K.S.; Lee, H.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol. Pharm. Bull. 2007, 30, 1674–1679. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Luong, T.T.; Kim, G.L.; Pyo, S.; Rhee, D.K. Korean Red Ginseng inhibits apoptosis in neuroblastoma cells via estrogen receptor beta-mediated phosphatidylinositol-3 kinase/Akt signaling. J. Ginseng Res. 2015, 39, 69–75. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gardlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.L.; Ha, T.; Li, C.; Kalbfleisch, J.H.; Schweitzer, J.; Vogt, W.; Browder, I.W. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit. Care Med. 2003, 31, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, M.; Park, E.K.; Kim, J.-S.; Hahn, D.; Bae, J.-S. Inhibitory Functions of Novel Compounds from Dioscorea batatas Decne Peel on HMGB1-mediated Septic Responses. Biotechnol. Bioprocess Eng. 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Lee, I.-C.; Ryu, C.-W.; Bae, J.-S. Novel Herbal Medicine C-KOK Suppresses the Inflammatory Gene iNOS via the Inhibition of p-STAT-1 and NF-κB. Biotechnol. Bioprocess Eng. 2020, 25, 536–542. [Google Scholar] [CrossRef]
- Lee, W.H.; Choo, S.; Sim, H.; Bae, J.S. Inhibitory Activities of Ononin on Particulate Matter-induced Oxidative Stress. Biotechnol. Bioprocess Eng. 2021, 26, 208–215. [Google Scholar] [CrossRef]
- Lee, W.; Ahn, J.H.; Park, H.H.; Kim, H.N.; Kim, H.; Yoo, Y.; Shin, H.; Hong, K.S.; Jang, J.G.; Park, C.G.; et al. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct. Target. Ther. 2020, 5, 186. [Google Scholar] [CrossRef]
- Lee, W.; Jeong, G.S.; Baek, M.C.; Ku, S.K.; Bae, J.S. Renal protective effects of aloin in a mouse model of sepsis. Food Chem. Toxicol. 2019, 132, 110651. [Google Scholar] [CrossRef]
- Lee, I.C.; Bae, J.S. Pelargonidin Protects Against Renal Injury in a Mouse Model of Sepsis. J. Med. Food 2019, 22, 57–61. [Google Scholar] [CrossRef]
- Sim, H.; Noh, Y.; Choo, S.; Kim, N.; Lee, T.; Bae, J.S. Suppressive Activities of Fisetin on Particulate Matter-induced Oxidative Stress. Biotechnol. Bioprocess Eng. 2021, 26, 568–574. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.O.; Kim, N.; Song, G.Y.; Bae, J.-S. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2022, 23, 10836. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810836
Kim GO, Kim N, Song GY, Bae J-S. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. International Journal of Molecular Sciences. 2022; 23(18):10836. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810836
Chicago/Turabian StyleKim, Go Oun, Nayeon Kim, Gyu Yong Song, and Jong-Sup Bae. 2022. "Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis" International Journal of Molecular Sciences 23, no. 18: 10836. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms231810836