A Proof-of-Concept Preclinical Study Using a Novel Thermal Insulation Device in a Porcine Kidney Auto-Transplantation Model
Abstract
:1. Introduction
2. Results
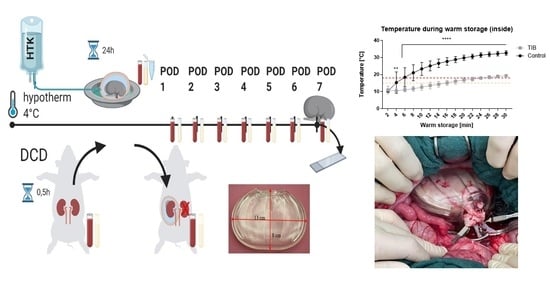
2.1. Ex Vivo—Testing
2.2. In Vivo Testing
2.2.1. Applicability, Surgical Management
2.2.2. Serum Values, Clinical Outcome
2.2.3. Thermographic Imaging
2.2.4. Histopathological Evaluation
2.2.5. ELISA
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Experimental Study Design
4.3. Histology
4.4. Data Analysis
4.5. Thermal Insulation Bag
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wadewitz, J. Grafiken Zum Tätigkeitsbericht 2020—Veröffentlicht Durch Die Deutsche Stiftung Organtransplantation. 2021. Available online: https://dso.de/BerichteTransplantationszentren/Grafiken%20D%202020%20Lunge.pdf (accessed on 10 October 2022).
- Lepoittevin, M.; Giraud, S.; Kerforne, T.; Barrou, B.; Badet, L.; Bucur, P.; Salamé, E.; Goumard, C.; Savier, E.; Branchereau, J.; et al. Preservation of Organs to Be Transplanted: An Essential Step in the Transplant Process. Int. J. Mol. Sci. 2022, 23, 4989. [Google Scholar] [CrossRef]
- Meister, F.A.; Czigany, Z.; Rietzler, K.; Miller, H.; Reichelt, S.; Liu, W.J.; Boecker, J.; Moeller, M.J.; Tolba, R.H.; Hamesch, K.; et al. Decrease of renal resistance during hypothermic oxygenated machine perfusion is associated with early allograft function in extended criteria donation kidney transplantation. Sci. Rep. 2020, 10, 17726. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Roselló-Catafau, J. Molecular Mechanisms and Pathophysiology of Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2018, 19, 4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, A.M.; Yuen, L.; Pang, T.; Rogers, N.; Hawthorne, W.J.; Pleass, H.C. Techniques to Ameliorate the Impact of Second Warm Ischemic Time on Kidney Transplantation Outcomes. Transplant. Proc. 2018, 50, 3144–3151. [Google Scholar] [CrossRef] [PubMed]
- Golriz, M.; Fonouni, H.; Kuttymuratov, G.; Esmaeilzadeh, M.; Rad, M.T.; Jarahian, P.; Longerich, T.; Faridar, A.; Abbasi, S.; Mehrabi, A.; et al. Influence of a modified preservation solution in kidney transplantation: A comparative experimental study in a porcine model. Asian J. Surg. 2017, 40, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Urbanellis, P.; Hamar, M.; Kaths, J.M.; Kollmann, D.; Linares, I.; Mazilescu, L.; Ganesh, S.; Wiebe, A.; Yip, P.M.; John, R.; et al. Normothermic Ex Vivo Kidney Perfusion Improves Early DCD Graft Function Compared with Hypothermic Machine Perfusion and Static Cold Storage. Transplantation 2020, 104, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Paller, M.S. Free radical-mediated postischemic injury in renal transplantation. Ren. Fail. 1992, 14, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, D.; Kościelska-Kasprzak, K.; Chudoba, P.; Hałoń, A.; Mazanowska, O.; Gomółkiewicz, A.; Dzięgiel, P.; Drulis-Fajdasz, D.; Myszka, M.; Lepiesza, A.; et al. The influence of warm ischemia elimination on kidney injury during transplantation—Clinical and molecular study. Sci. Rep. 2016, 6, 36118. [Google Scholar] [CrossRef] [Green Version]
- Kukla, U.; Cholewa, H.; Chronowska, J.; Goc, T.; Lieber, E.; Kolonko, A.; Budziński, G.; Ziaja, J.; Więcek, A.; Cierpka, L. Effect of the Second Warm Ischemia Time and Its Components on Early and Long-term Kidney Graft Function. Transplant. Proc. 2016, 48, 1365–1369. [Google Scholar] [CrossRef]
- Tennankore, K.K.; Kim, S.J.; Alwayn, I.P.; Kiberd, B.A. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016, 89, 648–658. [Google Scholar] [CrossRef]
- Lepiesza, A.; Chudoba, P.; Kamińska, D.; Pupka, A.; Zaleska, P. Methods of Reduction of Warm Ischemic Time in Kidney Transplantation and Their Role of Early and Late Outcomes. Polim. Med. 2016, 46, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karipineni, F.; Campos, S.; Parsikia, A.; Durinka, J.B.; Chang, P.N.; Khanmoradi, K.; Zaki, R.; Ortiz, J. Elimination of warm ischemia using the Ice Bag Technique does not decrease delayed graft function. Int. J. Surg. 2014, 12, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.; Kwarcinski, J.; Pang, T.; Hameed, A.; Boughton, P.; O’Grady, G.; Hawthorne, W.J.; Rogers, N.M.; Wong, G.; Pleass, H.C. Protection from the Second Warm Ischemic Injury in Kidney Transplantation Using an Ex Vivo Porcine Model and Thermally Insulating Jackets. Transplant. Proc. 2021, 53, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Torai, S.; Yoshimoto, S.; Yoshioka, M.; Nadahara, S.; Kobayashi, E. Reduction of Warm Ischemia Using a Thermal Barrier Bag in Kidney Transplantation: Study in a Pig Model. Transplant. Proc. 2019, 51, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Torai, S.; Kobayashi, E. The global trend for development of organ protection device to reduce secondary warm ischemic injury. Organ Biol. 2022, 29, 46–52. [Google Scholar]
- Meier, R.P.H.; Piller, V.; Hagen, M.E.; Joliat, C.; Buchs, J.B.; Nastasi, A.; Ruttimann, R.; Buchs, N.C.; Moll, S.; Vallée, J.P.; et al. Intra-Abdominal Cooling System Limits Ischemia-Reperfusion Injury During Robot-Assisted Renal Transplantation. Am. J. Transplant. 2018, 18, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Torai, S. Intra-abdominal Cooling System for the Transplanted Kidney. Transplant. Direct 2019, 5, e438. [Google Scholar] [CrossRef]
- Ferede, A.A.; Walsh, A.L.; Davis, N.F.; Smyth, G.; Mohan, P.; Power, R.; Forde, J.; O’Kelly, P.; Llittle, D. Warm Ischemia Time at Vascular Anastomosis is an Independent Predictor for Delayed Graft Function in Kidney Transplant Recipients. Exp. Clin. Transplant. 2020, 18, 13–18. [Google Scholar] [CrossRef]
- Kuipers, T.G.; Hellegering, J.; El Moumni, M.; Krikke, C.; Haveman, J.W.; Berger, S.P.; Leuvenink, H.G.; Pol, R.A. Kidney temperature course during living organ procurement and transplantation. Transpl. Int. 2017, 30, 162–169. [Google Scholar] [CrossRef]
- Heylen, L.; Pirenne, J.; Samuel, U.; Tieken, I.; Naesens, M.; Sprangers, B.; Jochmans, I. The Impact of Anastomosis Time During Kidney Transplantation on Graft Loss: A Eurotransplant Cohort Study. Am. J. Transplant. 2017, 17, 724–732. [Google Scholar] [CrossRef]
- Longchamp, A.; Meier, R.P.H.; Colucci, N.; Balaphas, A.; Orci, L.A.; Nastasi, A.; Longchamp, G.; Moll, S.; Klauser, A.; Pascual, M.; et al. Impact of an intra-abdominal cooling device during open kidney transplantation in pigs. Swiss Med. Wkly. 2019, 149, w20143. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Soh, S.; Kwak, Y.L.; Bae, J.C.; Kang, S.H.; Song, J.W. High Preoperative Serum Syndecan-1, a Marker of Endothelial Glycocalyx Degradation, and Severe Acute Kidney Injury after Valvular Heart Surgery. J. Clin. Med. 2020, 9, 1803. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- McGrath, J.C.; Drummond, G.; McLachlan, E.; Kilkenny, C.; Wainwright, C. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.J.; Ernst, L.; Doorschodt, B.; Bednarsch, J.; Becker, F.; Nakatake, R.; Masano, Y.; Neumann, U.P.; Lang, S.A.; Boor, P.; et al. Orthotopic Kidney Auto-Transplantation in a Porcine Model Using 24 Hours Organ Preservation and Continuous Telemetry. J. Vis. Exp. 2020, 162, e61591. [Google Scholar] [CrossRef] [PubMed]
- Klinge, U.; Dievernich, A.; Stegmaier, J. Quantitative Characterization of Macrophage, Lymphocyte, and Neutrophil Subtypes Within the Foreign Body Granuloma of Human Mesh Explants by 5-Marker Multiplex Fluorescence Microscopy. Front. Med. 2022, 9, 777439. [Google Scholar] [CrossRef]
- Swift, M.L. GraphPad prism, data analysis, and scientific graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]









| Group | Inflammation & Infiltration | Hemorrhage | Glomeruli Damage | Tubular Damage | Edema |
|---|---|---|---|---|---|
| DCD HTK | 2.9 ± 0.6 | 2.3 ± 0.9 | 2.2 ± 0.4 | 2.5 ± 0.6 | 2.4 ± 1.1 |
| DCD HTK TIB | 3.2 ± 1.2 | 2.8 ± 1.5 | 2.5 ± 0.6 | 2.7 ± 0.7 | 2.9 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, L.; Czigany, Z.; Paschenda, P.; Schulz, M.; Breuer, L.; Kunczik, J.; Czaplik, M.; Liu, W.; Jiang, D.; Klinge, U.; et al. A Proof-of-Concept Preclinical Study Using a Novel Thermal Insulation Device in a Porcine Kidney Auto-Transplantation Model. Int. J. Mol. Sci. 2022, 23, 13806. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232213806
Ernst L, Czigany Z, Paschenda P, Schulz M, Breuer L, Kunczik J, Czaplik M, Liu W, Jiang D, Klinge U, et al. A Proof-of-Concept Preclinical Study Using a Novel Thermal Insulation Device in a Porcine Kidney Auto-Transplantation Model. International Journal of Molecular Sciences. 2022; 23(22):13806. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232213806
Chicago/Turabian StyleErnst, Lisa, Zoltan Czigany, Pascal Paschenda, Mareike Schulz, Lukas Breuer, Janosch Kunczik, Michael Czaplik, Wenjia Liu, Decan Jiang, Uwe Klinge, and et al. 2022. "A Proof-of-Concept Preclinical Study Using a Novel Thermal Insulation Device in a Porcine Kidney Auto-Transplantation Model" International Journal of Molecular Sciences 23, no. 22: 13806. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232213806