Acute Exposure to Glycated Proteins Impaired in the Endothelium-Dependent Aortic Relaxation: A Matter of Oxidative Stress
Abstract
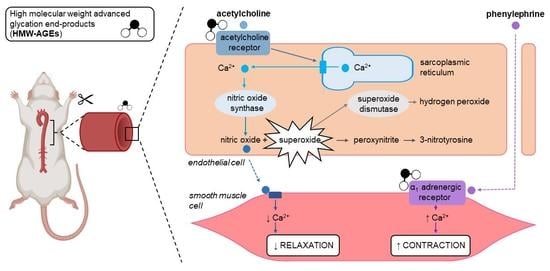
:1. Introduction
2. Results
2.1. Acute Exposure to HMW-AGEs Alters the Vasomotor Function
2.2. Preincubation with Superoxide Dismutase Improves Endothelium-Dependent Relaxation after Acute HMW-Ages Exposure
3. Discussion
3.1. Acute Exposure to HMW-AGEs Impairs Endothelium-Dependent Relaxation
3.2. The Impaired Endothelium-Dependent Relaxation Is Caused by Increased Superoxide Formation
3.3. Acute HMW-AGEs Exposure Tends to Increase Contractile Vascular Reactivity
4. Materials and Methods
4.1. Animal Experiments
4.2. Preparation and Protein Concentration Determination of HMW-AGEs
4.3. Assessment of Aortic Vasomotor Function
4.3.1. General Procedure
4.3.2. Vasorelaxation and Vasocontraction Responses
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twarda-Clapa, A.; Olczak, A.; Bialkowska, A.M.; Koziolkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Ayaz, A.; Buyuktuncer, Z. Formation of advanced glycation endproducts in foods during cooking process and underlying mechanisms: A comprehensive review of experimental studies. Nutr. Res. Rev. 2020, 33, 77–89. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2014, 3, 94–108. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [Green Version]
- Deluyker, D.; Evens, L.; Bito, V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: Focus on high molecular weight AGEs. Amino Acids 2017, 49, 1535–1541. [Google Scholar] [CrossRef]
- Gerdemann, A.; Lemke, H.D.; Nothdurft, A.; Heidland, A.; Munch, G.; Bahner, U.; Schinzel, R. Low-molecular but not high-molecular advanced glycation end products (AGEs) are removed by high-flux dialysis. Clin. Nephrol. 2000, 54, 276–283. [Google Scholar]
- Lamprea-Montealegre, J.A.; Arnold, A.M.; Mc, C.R.L.; Mukamal, K.J.; Djousse, L.; Biggs, M.L.; Siscovick, D.S.; Tracy, R.P.; Beisswenger, P.J.; Psaty, B.M.; et al. Plasma Levels of Advanced Glycation Endproducts and Risk of Cardiovascular Events: Findings From 2 Prospective Cohorts. J. Am. Heart Assoc. 2022, 11, e024012. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.S.; Ko, S.H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 2086. [Google Scholar] [CrossRef]
- Pinto, R.S.; Minanni, C.A.; de Araujo Lira, A.L.; Passarelli, M. Advanced Glycation End Products: A Sweet Flavor That Embitters Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 2404. [Google Scholar] [CrossRef] [PubMed]
- Bansode, S.B.; Gacche, R.N. Glycation-induced modification of tissue-specific ECM proteins: A pathophysiological mechanism in degenerative diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129411. [Google Scholar] [CrossRef] [PubMed]
- Daffu, G.; del Pozo, C.H.; O'Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Dinischiotu, A. RAGE and TGF-beta1 Cross-Talk Regulate Extracellular Matrix Turnover and Cytokine Synthesis in AGEs Exposed Fibroblast Cells. PLoS ONE 2016, 11, e0152376. [Google Scholar] [CrossRef] [PubMed]
- Engelen, L.; Stehouwer, C.D.; Schalkwijk, C.G. Current therapeutic interventions in the glycation pathway: Evidence from clinical studies. Diabetes Obes. Metab. 2013, 15, 677–689. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef]
- Miura, J.; Yamagishi, S.; Uchigata, Y.; Takeuchi, M.; Yamamoto, H.; Makita, Z.; Iwamoto, Y. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes. J. Diabetes Complicat. 2003, 17, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Penfold, S.A.; Coughlan, M.T.; Patel, S.K.; Srivastava, P.M.; Sourris, K.C.; Steer, D.; Webster, D.E.; Thomas, M.C.; MacIsaac, R.J.; Jerums, G.; et al. Circulating high-molecular-weight RAGE ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010, 78, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yu, W.; Zhang, B.; Cao, B.; Wang, M.; Hu, X. Distinctive effects of different types of advanced glycation end-products (AGEs) on liver glucose metabolism. Food Funct. 2022, 13, 11298–11306. [Google Scholar] [CrossRef]
- Deluyker, D.; Ferferieva, V.; Noben, J.P.; Swennen, Q.; Bronckaers, A.; Lambrichts, I.; Rigo, J.M.; Bito, V. Cross-linking versus RAGE: How do high molecular weight advanced glycation products induce cardiac dysfunction? Int. J. Cardiol. 2016, 210, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Deluyker, D.; Evens, L.; Haesen, S.; Driesen, R.B.; Kuster, D.; Verboven, M.; Belien, H.; van der Velden, J.; Lambrichts, I.; Bito, V. Glycolaldehyde-Derived High-Molecular-Weight Advanced Glycation End-Products Induce Cardiac Dysfunction through Structural and Functional Remodeling of Cardiomyocytes. Cell. Physiol. Biochem. 2020, 54, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Haesen, S.; Col, U.; Schurgers, W.; Evens, L.; Verboven, M.; Driesen, R.B.; Bronckaers, A.; Lambrichts, I.; Deluyker, D.; Bito, V. Glycolaldehyde-modified proteins cause adverse functional and structural aortic remodeling leading to cardiac pressure overload. Sci. Rep. 2020, 10, 12220. [Google Scholar] [CrossRef] [PubMed]
- Deluyker, D.; Evens, L.; Belien, H.; Bito, V. Acute exposure to glycated proteins reduces cardiomyocyte contractile capacity. Exp. Physiol. 2019, 104, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greven, W.L.; Smit, J.M.; Rommes, J.H.; Spronk, P.E. Accumulation of advanced glycation end (AGEs) products in intensive care patients: An observational, prospective study. BMC Clin. Pathol. 2010, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowenstein, C.J.; Dinerman, J.L.; Snyder, S.H. Nitric oxide: A physiologic messenger. Ann. Intern. Med. 1994, 120, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [PubMed]
- Bonaventura, D.; Lunardi, C.N.; Rodrigues, G.J.; Neto, M.A.; Bendhack, L.M. A novel mechanism of vascular relaxation induced by sodium nitroprusside in the isolated rat aorta. Nitric Oxide 2008, 18, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, M.S.; Nishtala, P.S. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm. J. 2017, 25, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, G.E.; Thompson, A.J. Evidence for an effect of receptor density on ligand occupancy and agonist EC50. Sci. Rep. 2019, 9, 19111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidasee, K.R.; Nallani, K.; Yu, Y.; Cocklin, R.R.; Zhang, Y.; Wang, M.; Dincer, U.D.; Besch, H.R., Jr. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 2003, 52, 1825–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R., Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 2004, 53, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Mao, N.; Li, M.; Dong, X.; Lin, F.Z.; Xu, Y.; Li, Y.B. KB-R7943 restores endothelium-dependent relaxation induced by advanced glycosylation end products in rat aorta. J. Diabetes Complicat. 2013, 27, 6–10. [Google Scholar] [CrossRef]
- Yin, Q.F.; Xiong, Y. Pravastatin restores DDAH activity and endothelium-dependent relaxation of rat aorta after exposure to glycated protein. J. Cardiovasc. Pharmacol. 2005, 45, 525–532. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Elberry, A.A.; Ghareib, S.A. Geraniol improves the impaired vascular reactivity in diabetes and metabolic syndrome through calcium channel blocking effect. J. Diabetes Complicat. 2016, 30, 1008–1016. [Google Scholar] [CrossRef]
- Tarkhan, M.M.; Balamsh, K.S.; El-Bassossy, H.M. Cinnamaldehyde protects from methylglyoxal-induced vascular damage: Effect on nitric oxide and advanced glycation end products. J. Food Biochem. 2019, 43, e12907. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.A.; El-Bassossy, H.M.; Azhar, A.S.; Tarkhan, M.M.; El-Mas, M.M. Interference with AGEs formation and AGEs-induced vascular injury mediates curcumin vascular protection in metabolic syndrome. Sci. Rep. 2020, 10, 315. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, C.M.; Pereira, A.M.; Seica, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Kawasaki, H.; Abe, K.; Kanno, M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am. J. Physiol. 1991, 261, H1086–H1094. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.B.; Somers, M.; Kurz, S.; McCann, L.; Warnholtz, A.; Freeman, B.A.; Tarpey, M.; Fukai, T.; Harrison, D.G. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001, 103, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryglewski, R.J.; Palmer, R.M.; Moncada, S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986, 320, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Mengstie, M.A.; Chekol Abebe, E.; Behaile Teklemariam, A.; Tilahun Mulu, A.; Agidew, M.M.; Teshome Azezew, M.; Zewde, E.A.; Agegnehu Teshome, A. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci. 2022, 9, 1002710. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Wautier, J.L.; Zoukourian, C.; Chappey, O.; Wautier, M.P.; Guillausseau, P.J.; Cao, R.; Hori, O.; Stern, D.; Schmidt, A.M. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J. Clin. Investig. 1996, 97, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Rojas, A.; Romay, S.; Gonzalez, D.; Herrera, B.; Delgado, R.; Otero, K. Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products. Circ. Res. 2000, 86, E50–E54. [Google Scholar] [CrossRef] [PubMed]
- Naser, N.; Januszewski, A.S.; Brown, B.E.; Jenkins, A.J.; Hill, M.A.; Murphy, T.V. Advanced glycation end products acutely impair Ca(2+) signaling in bovine aortic endothelial cells. Front. Physiol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potje, S.R.; Paula, T.D.; Paulo, M.; Bendhack, L.M. The Role of Glycocalyx and Caveolae in Vascular Homeostasis and Diseases. Front. Physiol. 2020, 11, 620840. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.B.; Feron, O.; Sacks, D.; Michel, T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997, 272, 15583–15586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegab, Z.; Mohamed, T.M.A.; Stafford, N.; Mamas, M.; Cartwright, E.J.; Oceandy, D. Advanced glycation end products reduce the calcium transient in cardiomyocytes by increasing production of reactive oxygen species and nitric oxide. FEBS Open Bio 2017, 7, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, R.R., Jr.; Nichols, A.J.; Stadel, J.M.; Hieble, J.P. Structure and function of alpha-adrenoceptors. Pharmacol. Rev. 1991, 43, 475–505. [Google Scholar] [PubMed]
- Sheng, Y.; Zhu, L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 17–28. [Google Scholar]
- Cunha, T.S.; Moura, M.J.; Bernardes, C.F.; Tanno, A.P.; Marcondes, F.K. Vascular sensitivity to phenylephrine in rats submitted to anaerobic training and nandrolone treatment. Hypertension 2005, 46, 1010–1015. [Google Scholar] [CrossRef] [Green Version]
- Palacios, J.; Vega, J.L.; Paredes, A.; Cifuentes, F. Effect of phenylephrine and endothelium on vasomotion in rat aorta involves potassium uptake. J. Physiol. Sci. 2013, 63, 103–111. [Google Scholar] [CrossRef]
- Eid, B.G.; Abu-Sharib, A.T.; El-Bassossy, H.M.; Balamash, K.; Smirnov, S.V. Enhanced calcium entry via activation of NOX/PKC underlies increased vasoconstriction induced by methylglyoxal. Biochem. Biophys. Res. Commun. 2018, 506, 1013–1018. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]


| Groups | Emax (%) | LogEC50 (M) | |
|---|---|---|---|
| ACh | Non-exposed | 85.09 ± 1.95 | −6.81 ± 0.07 |
| HMW-AGEs | 71.18 ± 2.98 * | −6.54 ± 0.07 * | |
| Non-exposed + SOD | 81.24 ± 4.04 | −7.01 ± 0.10 | |
| HMW-AGEs + SOD | 93.89 ± 1.50 × | −7.41 ± 0.07 × | |
| SNP | Non-exposed | 99.71 ± 0.05 | −8.39 ± 0.05 |
| HMW-AGEs | 99.23 ± 0.07 | −8.25 ± 0.05 | |
| PE | Non-exposed | 3.14 ± 0.14 | −7.14 ± 0.06 |
| HMW-AGEs | 3.29 ± 0.23 | −7.35 ± 0.11 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Haese, S.; Deluyker, D.; Bito, V. Acute Exposure to Glycated Proteins Impaired in the Endothelium-Dependent Aortic Relaxation: A Matter of Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 14916. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232314916
D’Haese S, Deluyker D, Bito V. Acute Exposure to Glycated Proteins Impaired in the Endothelium-Dependent Aortic Relaxation: A Matter of Oxidative Stress. International Journal of Molecular Sciences. 2022; 23(23):14916. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232314916
Chicago/Turabian StyleD’Haese, Sarah, Dorien Deluyker, and Virginie Bito. 2022. "Acute Exposure to Glycated Proteins Impaired in the Endothelium-Dependent Aortic Relaxation: A Matter of Oxidative Stress" International Journal of Molecular Sciences 23, no. 23: 14916. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232314916