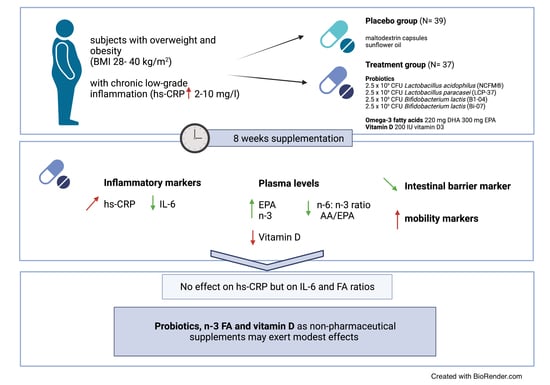
Potential Modulation of Inflammation and Physical Function by Combined Probiotics, Omega-3 Supplementation and Vitamin D Supplementation in Overweight/Obese Patients with Chronic Low-Grade Inflammation: A Randomized, Placebo-Controlled Trial
Abstract
:1. Introduction
1.1. Obesity and Low-Grade Inflammation
1.2. The Potential of Dietary Supplements
2. Results
2.1. Subject Characterization
2.2. Effect of Supplementation on hs-CRP Levels and other Inflammatory Markers
2.3. Laboratory Parameters
2.4. Fatty Acid Changes in Plasma
2.5. Physical Function Tests
2.6. Gut Health
2.6.1. Short-Chain Fatty Acids
2.6.2. Gut Barrier Markers
2.7. Correlation Analysis of All Data including All Participants
2.8. Subgroup Analysis
3. Discussion
4. Materials and Methods
4.1. Participant Selection
4.2. Study Design, Visits, Power Calculation and Outcome Parameters
4.2.1. Study Design
4.2.2. Power Calculations
4.2.3. Additional Outcome Parameters
4.2.4. Study Visits
4.3. Study Outcomes
4.3.1. Blood Plasma, Serum, Fecal, and Urine Collection and Measurements
4.3.2. Quantification of Plasma Fatty Acids
4.3.3. Inflammatory Markers and Multi-Sugar Urinary Recovery Test
4.3.4. Analysis of Intestinal Permeability Markers
4.3.5. Sit-to-Stand Test (SST), Western Ontario and McMaster Osteoarthritis Index (WOMAC), and Food Frequency Questionnaire (FFQ)
4.3.6. Study Products, Randomization, and Blinding
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 November 2022).
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- You, T.; Yang, R.; Lyles, M.F.; Gong, D.; Nicklas, B.J. Abdominal Adipose Tissue Cytokine Gene Expression: Relationship to Obesity and Metabolic Risk Factors. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E741–E747. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Hersoug, L.; Møller, P.; Loft, S. Role of Microbiota-Derived Lipopolysaccharide in Adipose Tissue Inflammation, Adipocyte Size and Pyroptosis during Obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The Role of Gut Microbiota in the Development of Obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The Microbiota-Gut-Brain Axis in Obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta BBA Mol. Cell. Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel Pro-Resolving Lipid Mediators in Inflammation Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Polus, A.; Zapala, B.; Razny, U.; Gielicz, A.; Kiec-Wilk, B.; Malczewska-Malec, M.; Sanak, M.; Childs, C.E.; Calder, P.C.; Dembinska-Kiec, A. Omega-3 Fatty Acid Supplementation Influences the Whole Blood Transcriptome in Women with Obesity, Associated with pro-Resolving Lipid Mediator Production. Biochim. Biophys. Acta 2016, 1861, 1746–1755. [Google Scholar] [CrossRef]
- Djuric, Z.; Turgeon, D.K.; Sen, A.; Ren, J.; Herman, K.; Ramaswamy, D.; Zhao, L.; Ruffin, M.T.; Normolle, D.P.; Smith, W.L.; et al. The Anti-Inflammatory Effect of Personalized Omega-3 Fatty Acid Dosing for Reducing Prostaglandin E2 in the Colonic Mucosa Is Attenuated in Obesity. Cancer Prev. Res. 2017, 10, 729–737. [Google Scholar] [CrossRef]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with Eicosapentaenoic Acid and Docosahexaenoic Acid Reduces High Levels of Circulating Proinflammatory Cytokines in Aging Adults: A Randomized, Controlled Study. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 23–29. [Google Scholar] [CrossRef]
- Majewska, K.; Kręgielska-Narożna, M.; Jakubowski, H.; Szulińska, M.; Bogdański, P. The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study. J. Clin. Med. 2020, 9, 998. [Google Scholar] [CrossRef]
- Maia, L.P.; de Almeida Silva Levi, Y.L.; do Prado, R.L.; dos Santos Santinoni, C.; Marsicano, J.A. Effects of Probiotic Therapy on Serum Inflammatory Markers: A Systematic Review and Meta-Analysis. J. Funct. Foods 2019, 54, 466–478. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium Strains and Galactooligosaccharides Improve Intestinal Barrier Function in Obese Adults but Show No Synergism When Used Together as Synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus Paracasei Modulates the Gut Microbiota and Improves Inflammation in Type 2 Diabetic Rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; Alfieri, D.F.; Lozovoy, M.A.B.; Mari, N.L.; de Souza, C.H.B.; Dichi, I.; Costa, G.N. Beneficial Effects of Bifidobacterium Lactis on Lipid Profile and Cytokines in Patients with Metabolic Syndrome: A Randomized Trial. Effects of Probiotics on Metabolic Syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and Elderly Women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’ai, H.; Patimah, I.; Rahmat, A.; Abed, Y. Effect of Long Chain Omega-3 Polyunsaturated Fatty Acids on Inflammation and Metabolic Markers in Hypertensive and/or Diabetic Obese Adults: A Randomized Controlled Trial. Food Nutr. Res. 2016, 60, 29268. [Google Scholar] [CrossRef]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D Supplementation for Improvement of Chronic Low-Grade Inflammation in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of Probiotic (VSL#3) and Omega-3 on Lipid Profile, Insulin Sensitivity, Inflammatory Markers, and Gut Colonization in Overweight Adults: A Randomized, Controlled Trial. Mediat. Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef]
- Tingö, L.; Hutchinson, A.N.; Bergh, C.; Stiefvatter, L.; Schweinlin, A.; Jensen, M.G.; Krüger, K.; Bischoff, S.C.; Brummer, R.J. Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial. Nutrients 2022, 14, 3998. [Google Scholar] [CrossRef]
- Costanzo, M.; Cesi, V.; Palone, F.; Pierdomenico, M.; Colantoni, E.; Leter, B.; Vitali, R.; Negroni, A.; Cucchiara, S.; Stronati, L. Krill Oil, Vitamin D and Lactobacillus Reuteri Cooperate to Reduce Gut Inflammation. Benef. Microbes 2018, 9, 389–399. [Google Scholar] [CrossRef]
- Brennan Laing, B.; Cavadino, A.; Ellett, S.; Ferguson, L.R. Effects of an Omega-3 and Vitamin D Supplement on Fatty Acids and Vitamin D Serum Levels in Double-Blinded, Randomized, Controlled Trials in Healthy and Crohn’s Disease Populations. Nutrients 2020, 12, 1139. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-Based Nutritional and Pharmacological Interventions Targeting Chronic Low-Grade Inflammation in Middle-Age and Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef]
- Khaodhiar, L.; Ling, P.-R.; Blackburn, G.L.; Bistrian, B.R. Serum Levels of Interleukin-6 and C-Reactive Protein Correlate With Body Mass Index Across the Broad Range of Obesity. J. Parenter. Enter. Nutr. 2004, 28, 410–415. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-Reactive Protein in Various Populations: A Systematic Review and Meta-Analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef]
- CRP at Early Follicular Phase of Menstrual Cycle Can Cause Misinterpretation for Cardiovascular Risk Assessment—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26767119/ (accessed on 18 May 2022).
- Gaskins, A.J.; Wilchesky, M.; Mumford, S.L.; Whitcomb, B.W.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Endogenous Reproductive Hormones and C-Reactive Protein across the Menstrual Cycle: The BioCycle Study. Am. J. Epidemiol. 2012, 175, 423–431. [Google Scholar] [CrossRef]
- Blum, C.A.; Müller, B.; Huber, P.; Kraenzlin, M.; Schindler, C.; De Geyter, C.; Keller, U.; Puder, J.J. Low-Grade Inflammation and Estimates of Insulin Resistance during the Menstrual Cycle in Lean and Overweight Women. J. Clin. Endocrinol. Metab. 2005, 90, 3230–3235. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Micallef, M.A.; Munro, I.A.; Garg, M.L. An Inverse Relationship between Plasma N-3 Fatty Acids and C-Reactive Protein in Healthy Individuals. Eur. J. Clin. Nutr. 2009, 63, 1154–1156. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Zhao, Y.; Soares, M.J. The Impact of Cholecalciferol Supplementation on the Systemic Inflammatory Profile: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials. Eur. J. Clin. Nutr. 2017, 71, 931–943. [Google Scholar] [CrossRef]
- Nasermoaddeli, A.; Sekine, M.; Kagamimori, S. Intra-Individual Variability of High-Sensitivity C-Reactive Protein. Circ. J. 2006, 70, 559–563. [Google Scholar] [CrossRef]
- Bower, J.K.; Lazo, M.; Juraschek, S.P.; Selvin, E. Within-Person Variability in High-Sensitivity C-Reactive Protein. Arch. Intern. Med. 2012, 172, 1519–1521. [Google Scholar] [CrossRef]
- Hage, F.G.; Szalai, A.J. C-Reactive Protein Gene Polymorphisms, C-Reactive Protein Blood Levels, and Cardiovascular Disease Risk. J. Am. Coll. Cardiol. 2007, 50, 1115–1122. [Google Scholar] [CrossRef]
- Kushner, I.; Rzewnicki, D.; Samols, D. What Does Minor Elevation of C-Reactive Protein Signify? Am. J. Med. 2006, 119, 166.e17–166.e28. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Variya, B.; Ilangumaran, S.; Langlois, M.-F.; Ramanathan, S. Inflammation in Human Adipose Tissues-Shades of Gray, Rather than White and Brown. Cytokine Growth Factor. Rev. 2018, 44, 28–37. [Google Scholar] [CrossRef]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The Major Inflammatory Mediator Interleukin-6 and Obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Zammit, V.A.; Park, S.-O. Molecular Mechanism for Hepatic Glycerolipid Partitioning of N-6/n-3 Fatty Acid Ratio in an Obese Animal Biomodels. Int. J. Mol. Sci. 2023, 24, 1576. [Google Scholar] [CrossRef]
- Guerendiain, M.; Montes, R.; López-Belmonte, G.; Martín-Matillas, M.; Castellote, A.I.; Martín-Bautista, E.; Martí, A.; Martínez, J.A.; Moreno, L.; Garagorri, J.M.; et al. Changes in Plasma Fatty Acid Composition Are Associated with Improvements in Obesity and Related Metabolic Disorders: A Therapeutic Approach to Overweight Adolescents. Clin. Nutr. 2018, 37, 149–156. [Google Scholar] [CrossRef]
- Sugano, M.; Hirahara, F. Polyunsaturated Fatty Acids in the Food Chain in Japan. Am. J. Clin. Nutr. 2000, 71, 189S–196S. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Kawauchi, K.; Atsumi, W.; Matsuo, R.; Ashida, T.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A.; Matsumoto, N.; et al. Association among Daily Fish Intake, White Blood Cell Count, and Healthy Lifestyle Behaviors in an Apparently Healthy Japanese Population: Implication for the Anti-Atherosclerotic Effect of Fish Consumption. Heart Vessel. 2021, 36, 924–933. [Google Scholar] [CrossRef] [PubMed]
- The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases—Artemis P. Simopoulos. 2008. Available online: https://0-journals-sagepub-com.brum.beds.ac.uk/doi/10.3181/0711-MR-311 (accessed on 13 May 2022).
- Simopoulos, A.P.; DiNicolantonio, J.J. The Importance of a Balanced ω-6 to ω-3 Ratio in the Prevention and Management of Obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Leslie, M.; Moghadasian, M.H.; Arendt, B.M.; Allard, J.P.; Ma, D.W.L. The Role of n − 6 and n − 3 Polyunsaturated Fatty Acids in the Manifestation of the Metabolic Syndrome in Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease. Food Funct. 2014, 5, 426–435. [Google Scholar] [CrossRef]
- Ito, R.; Satoh-Asahara, N.; Yamakage, H.; Sasaki, Y.; Odori, S.; Kono, S.; Wada, H.; Suganami, T.; Ogawa, Y.; Hasegawa, K.; et al. An Increase in the EPA/AA Ratio Is Associated with Improved Arterial Stiffness in Obese Patients with Dyslipidemia. JAT 2014, 21, 248–260. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of Marine-Derived n-3 Polyunsaturated Fatty Acids on C-Reactive Protein, Interleukin 6 and Tumor Necrosis Factor α: A Meta-Analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef]
- Weight Loss is a Critical Factor to Reduce Inflammation—ScienceDirect. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/abs/pii/S2405457718303954?via%3Dihub (accessed on 13 May 2022).
- Perna, S.; Ilyas, Z.; Giacosa, A.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Rigon, C.; Naso, M.; Riva, A.; Petrangolini, G.; et al. Is Probiotic Supplementation Useful for the Management of Body Weight and Other Anthropometric Measures in Adults Affected by Overweight and Obesity with Metabolic Related Diseases? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 666. [Google Scholar] [CrossRef]
- Hill, C.L.; March, L.M.; Aitken, D.; Lester, S.E.; Battersby, R.; Hynes, K.; Fedorova, T.; Proudman, S.M.; James, M.; Cleland, L.G.; et al. Fish Oil in Knee Osteoarthritis: A Randomised Clinical Trial of Low Dose versus High Dose. Ann. Rheum. Dis. 2016, 75, 23–29. [Google Scholar] [CrossRef]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The Effect of Probiotic Lactobacillus Casei Shirota on Knee Osteoarthritis: A Randomised Double-Blind, Placebo-Controlled Clinical Trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef]
- Gao, X.-R.; Chen, Y.-S.; Deng, W. The Effect of Vitamin D Supplementation on Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Int. J. Surg. 2017, 46, 14–20. [Google Scholar] [CrossRef]
- Messier, S.P.; Beavers, D.P.; Mihalko, S.L.; Miller, G.D.; Lyles, M.F.; Hunter, D.J.; Carr, J.J.; Eckstein, F.; Guermazi, A.; Loeser, R.F.; et al. The Effects of Intensive Dietary Weight Loss and Exercise on Gait in Overweight and Obese Adults with Knee Osteoarthritis. The Intensive Diet and Exercise for Arthritis (IDEA) Trial. J. Biomech. 2020, 98, 109477. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, D.M.; Garruti, G.; Di Ciaula, A.; Molina-Molina, E.; Shanmugam, H.; De Angelis, M.; Portincasa, P. Increased Colonic Permeability and Lifestyles as Contributing Factors to Obesity and Liver Steatosis. Nutrients 2020, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in Health and Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Rieckmann, N.; Hintzpeter, B.; Mensink, G.B.M. Vitamin D Status among Adults in Germany—Results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015, 15, 641. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Frick, K.; Lehnert, K.; Vetter, W.; Montoya-Arroyo, A.; Frank, J.; Schmid-Staiger, U.; Bischoff, S.C. Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum Tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals. Mar. Drugs 2022, 20, 716. [Google Scholar] [CrossRef]
- Thurnhofer, S.; Lehnert, K.; Vetter, W. Exclusive Quantification of Methyl-Branched Fatty Acids and Minor 18:1-Isomers in Foodstuff by GC/MS in the SIM Mode Using 10,11-Dichloroundecanoic Acid and Fatty Acid Ethyl Esters as Internal Standards. Eur. Food Res. Technol. 2008, 226, 975–983. [Google Scholar] [CrossRef]
- Thurnhofer, S.; Vetter, W. Application of Ethyl Esters and D3-Methyl Esters as Internal Standards for the Gas Chromatographic Quantification of Transesterified Fatty Acid Methyl Esters in Food. J. Agric. Food Chem. 2006, 54, 3209–3214. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum Tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef]
- McConnell, S.; Kolopack, P.; Davis, A.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A Review of Its Utility and Measurement Properties. Arthritis Care Res. 2001, 45, 453–461. [Google Scholar] [CrossRef]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative Validation of a Food Frequency Questionnaire for National Health and Nutrition Monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef]




| Placebo (n = 39) | Treatment (n = 37) | p-Value | |
|---|---|---|---|
| Women/men (n) | 30/9 | 30/7 | |
| Anthropometry | |||
| Age [years] | 49 ± 10 | 50 ± 11 | 0.695 |
| Weight [kg] | 97.6 ± 12.3 | 100.9 ± 14 | 0.276 |
| BMI [kg/m2] | 33.8 ± 3.6 | 35.0 ± 2.9 | 0.133 |
| n overweight | 8 | 0 | |
| n obese | 31 | 37 | |
| Blood biomarkers | |||
| hs-CRP [mg/L] | 4.1 ± 2.0 | 4.4 ± 2.1 | 0.433 |
| γ-GT [U/L] | 33 ± 24 | 29 ± 15 | 0.578 |
| AST [U/L] | 30 ± 31 | 26 ± 11 | 0.932 |
| ALT [U/L] | 28 ± 17 | 29 ± 14 | 0.445 |
| Plasma glucose [mg/dL] | 90 ± 10 | 95 ± 14 | 0.123 |
| Insulin [µE/mL] | 19.0 ± 15.7 | 25.2 ± 28.5 | 0.856 |
| HOMA index | 4.2 ± 3.5 | 6.2 ± 7.8 | 0.735 |
| Creatinine [mg/dL] | 0.83 ± 0.15 | 0.79 ± 0.15 | 0.253 |
| Uric acid [mg/dL] | 5.9 ± 1.4 | 5.6 ± 1.1 | 0.295 |
| Cholesterol [mg/dL] | 236 ± 53 | 231 ± 42 | 0.660 |
| Triglycerides [mg/dL] | 169 ± 78 | 148 ± 64 | 0.257 |
| HDL cholesterol [mg/dL] | 52 ± 12 | 53 ± 11 | 0.294 |
| LDL cholesterol [mg/dL] | 154 ± 39 | 150 ± 30 | 0.619 |
| LDL/HDL cholesterol quotient | 3.1 ± 0.9 | 2.9 ± 0.7 | 0.367 |
| FFQ | |||
| Energy [kcal] | 1904.0 ± 867.2 | 1816.0 ± 896.4 | 0.560 |
| Fat [g] | 75.4 ± 41.6 | 75.3 ± 40.0 | 0.890 |
| Saturated fatty acids [g] | 34.6 ± 20.4 | 36.0 ± 20.8 | 0.687 |
| Polyunsaturated fatty acids [g] | 10.8 ± 5.8 | 9.5 ± 5.0 | 0.299 |
| Short chain fatty acids [g] | 1.7 ± 1.2 | 1.9 ± 1.2 | 0.641 |
| Vitamin D [μg] | 4.2 ± 3.2 | 3.8 ± 2.5 | 0.832 |
| Placebo | Treatment Group | Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| W0 | W8 | ΔW8 − W0 Treatment Effect | W0 | W8 | ΔW8 − W0 Treatment Effect | p-Value | p-Value | |
| W8 | ΔW8 − W0 Treatment Effect | |||||||
| Inflammatory markers | ||||||||
| Hs-CRP [mg/L] | 4.4 ± 3.9 | 4.9 ± 3.6 | 0.51 ± 4.84 | 4.2 ± 2.4 | 5.5 * ± 3.8 | 1.34 ± 3.31 | 0.394 | 0.159 |
| IL-6 [pg/mL] | 0.8 ± 0.5 | 0.9 ± 0.6 | 0.08 ± 0.37 | 1.0 ± 0.9 | 0.9 * ± 0.8 | −0.04 ± 0.62 | 0.477 | 0.848 |
| TNF-α [pg/mL] | 1.4 ± 0.7 | 1.4 ± 0.8 | 0.08 ± 0.33 | 1.3 ± 0.6 | 1.4 ± 0.7 | 0.06 ± 0.27 | 0.666 | 0.326 |
| IFN-γ [pg/mL] | 5.0 ± 2.7 | 6.3 ± 5.3 | 1.33 ± 5.44 | 5.3 ± 4.4 | 5.4 ± 4.1 | 0.10 ± 2.85 | 0.287 | 0.26 |
| IL-4 [pg/mL] | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.00 ± 0.01 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.00 ± 0.02 | 0.477 | 0.427 |
| IL-8 [pg/mL] | 11.4 ± 6.3 | 11.5 ± 5.7 | 0.03 ± 2.48 | 19.9 ± 43.8 | 19.6 ± 39.2 | −0.38 ± 5.46 | 0.856 | 0.856 |
| IL-12 [pg/mL] | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.02 ± 0.16 | 0.2 ± 0.6 | 0.2 ± 0.6 | 0.02 ± 0.09 | 0.249 | 0.86 |
| IL-10 [pg/mL] | 0.3 ± 0.4 | 0.3 ± 0.3 | 0.03 ± 0.22 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.07 | 0.131 | 0.629 |
| Laboratory Parameters | ||||||||
| Weight [kg] | 97.2 ± 12.1 | 97.4 ± 12.1 | 0.15 ± 2.02 | 101.1 ± 14.3 | 100.5 ± 14.2 | 0.17 ± 2.28 | 0.316 | 0.766 |
| BMI [kg/m2] | 33.7 ± 3.5 | 33.8 ± 3.5 | 0.06 ± 0.7 | 35.0 ± 2.8 | 35.1 ± 2.7 | 0.05 ± 0.78 | 0.083 | 0.812 |
| Vitamin D [ng/mL] | 22.4 ± 11.8 | 22.1 ± 10.6 | −0.33 ± 5.85 | 24.1 ± 15.7 | 24.6 ± 10.9 | 0.51 ± 8.05 | 0.259 | 0.359 |
| Glucose [mg/dL] | 91 ± 10 | 93 * ± 12 | 1.97 ± 6.27 | 94 ± 12 | 94 ± 13 | 0.32 ± 6.28 | 0.505 | 0.256 |
| Insulin [µE/mL] | 14.8 ± 7.5 | 14.9 ± 7.3 | 0.04 ± 5.14 | 15.2 ± 8.5 | 14.7 ± 7.7 | −0.4 ± 5.07 | 0.687 | 0.664 |
| HOMA index | 3.3 ± 1.8 | 3.4 ± 1.8 | 0.06 ± 1.35 | 3.5 ± 2.1 | 3.5 ± 1.9 | 0.02 ± 1.44 | 0.903 | 0.738 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopp, L.; Schweinlin, A.; Tingö, L.; Hutchinson, A.N.; Feit, V.; Jähnichen, T.; Lehnert, K.; Vetter, W.; Rings, A.; Jensen, M.G.; et al. Potential Modulation of Inflammation and Physical Function by Combined Probiotics, Omega-3 Supplementation and Vitamin D Supplementation in Overweight/Obese Patients with Chronic Low-Grade Inflammation: A Randomized, Placebo-Controlled Trial. Int. J. Mol. Sci. 2023, 24, 8567. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24108567
Kopp L, Schweinlin A, Tingö L, Hutchinson AN, Feit V, Jähnichen T, Lehnert K, Vetter W, Rings A, Jensen MG, et al. Potential Modulation of Inflammation and Physical Function by Combined Probiotics, Omega-3 Supplementation and Vitamin D Supplementation in Overweight/Obese Patients with Chronic Low-Grade Inflammation: A Randomized, Placebo-Controlled Trial. International Journal of Molecular Sciences. 2023; 24(10):8567. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24108567
Chicago/Turabian StyleKopp, Lena, Anna Schweinlin, Lina Tingö, Ashley N. Hutchinson, Viktoria Feit, Tabea Jähnichen, Katja Lehnert, Walter Vetter, Andreas Rings, Morten G. Jensen, and et al. 2023. "Potential Modulation of Inflammation and Physical Function by Combined Probiotics, Omega-3 Supplementation and Vitamin D Supplementation in Overweight/Obese Patients with Chronic Low-Grade Inflammation: A Randomized, Placebo-Controlled Trial" International Journal of Molecular Sciences 24, no. 10: 8567. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24108567