Selective Encapsulation of the Polyphenols on Silk Fibroin Nanoparticles: Optimization Approaches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Silk Fibroin-Based Nanoparticles
2.2. Characterization of the Phenolic Compound-Based Mixture
2.3. Effect of Silk Fibroin and Ethanol Concentration on Average Particle Size
2.4. Effect of Silk Fibroin and Ethanol Concentration on Encapsulated Phenolic Compound Concentration
2.5. Effect of pH on the on Encapsulated Phenolic Compound Concentration
2.6. Determination of Effect of Phenolic Compounds on the Selective Encapsulation
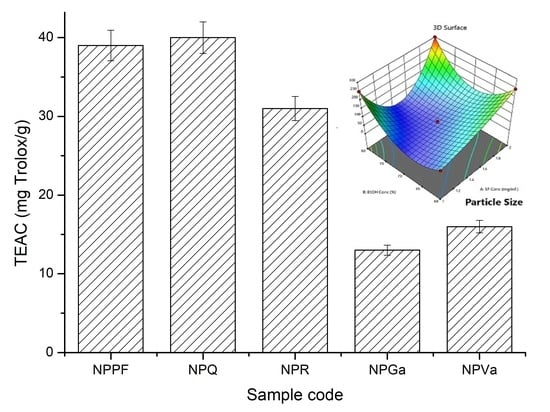
2.7. Thin-Layer Chromatography (TLC) and Antioxidant Activity of the Selective Encapsulated Polyphenols
3. Materials and Methods
3.1. Materials
3.2. Preparation Methods
3.2.1. Preparation of Silk Fibroin Solution
3.2.2. Preparation of Phenolic Compounds Mix
3.2.3. Preparation of Silk Fibroin Nanoparticles
3.2.4. Preparation of Silk Fibroin Nanoparticles Loaded with Bioactive Compounds
3.3. Characterization Methods
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.3.2. HPLC Analysis
3.3.3. Experimental Design
3.3.4. Particle Size Analysis
3.3.5. Thin-Layer Chromatographic Chemical Screening (TLC)
3.3.6. ABTS Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahya, N.A.; Attan, N.; Wahab, R.A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018, 112, 69–85. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.D.; Bayraktar, O. Encapsulated Plant-Derived Polyphenols as Potential Cancer Treatment Agents. In Herbal Medicine: Back to the Future Cancer Therapy; Murad, F., Rahman, A., Bian, K., Eds.; Bentham Science: Amsterdam, The Netherlands, 2019; pp. 91–122. [Google Scholar]
- Bayraktar, O.; Erdoğan, I.; Köse, M.D.; Kalmaz, G. Nanocarriers for Plant-Derived Natural Compounds. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 395–410. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Kma, L. Plant Extracts and Plant-Derived Compounds: Promising Players in Countermeasure Strategy Against Radiological Exposure: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 2405–2425. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. An Introduction to Natural Products Isolation. Methods Mol. Biol. 2012, 864, 1–25. [Google Scholar] [CrossRef]
- Comunian, T.A.; Anthero, A.G.D.S.; Bezerra, E.O.; Moraes, I.C.F.; Hubinger, M.D. Encapsulation of Pomegranate Seed Oil by Emulsification Followed by Spray Drying: Evaluation of Different Biopolymers and Their Effect on Particle Properties. Food Bioprocess Technol. 2019, 13, 53–66. [Google Scholar] [CrossRef]
- Tavares, L.; Noreña, C.P.Z. Encapsulation of Ginger Essential Oil Using Complex Coacervation Method: Coacervate Formation, Rheological Property, and Physicochemical Characterization. Food Bioprocess Technol. 2020, 13, 1405–1420. [Google Scholar] [CrossRef]
- Bayraktar, O.; Yahsi, Y.; Köse, M.D. Electroencapsulation of Trans-resveratrol in Nanoparticles Composed of Silk Fibroin and Soluble Eggshell Membrane Protein. Food Bioprocess Technol. 2021, 14, 334–351. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk Fibroin-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef]
- Shi, P.; Goh, J.C. Self-assembled silk fibroin particles: Tunable size and appearance. Powder Technol. 2012, 215–216, 85–90. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, A.; Li, Y.; Hu, J.; Liu, X.; Li, J.; Zhang, Y.; Li, G.; Zheng, Z. Fabrication of silk fibroin nanoparticles for controlled drug delivery. J. Nanoparticle Res. 2012, 14, 736. [Google Scholar] [CrossRef]
- Basal, G.; Altiok, D.; Bayraktar, O. Antibacterial properties of silk fibroin/chitosan blend films loaded with plant extract. Fibers Polym. 2010, 11, 21–27. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- McGill, M.; Coburn, J.M.; Partlow, B.P.; Mu, X.; Kaplan, D.L. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 2017, 63, 76–84. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef]
- Calamak, S.; Aksoy, E.A.; Erdogdu, C.; Sagıroglu, M.; Ulubayram, K. Silver nanoparticle containing silk fibroin bionanotextiles. J. Nanoparticle Res. 2015, 17, 87. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Shen, W.-D.; Xiang, R.-L.; Zhuge, L.-J.; Gao, W.-J.; Wang, W.-B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanoparticle Res. 2006, 9, 885–900. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef]
- Jun, J.Y.; Nguyen, H.H.; Paik, S.-Y.; Chun, H.S.; Kang, B.-C.; Ko, S. Preparation of size-controlled bovine serum albumin (BSA) nanoparticles by a modified desolvation method. Food Chem. 2011, 127, 1892–1898. [Google Scholar] [CrossRef]
- Azarmi, S.; Huang, Y.; Chen, H.; McQuarrie, S.; Abrams, D.; Roa, W.; Finlay, W.H.; Miller, G.G.; Löbenberg, R. Optimization of a two-step desolvation method for preparing gelatin nanoparticles and cell uptake studies in 143B osteosarcoma cancer cells. J. Pharm. Pharm. Sci. 2006, 9, 124–132. [Google Scholar] [PubMed]
- Wang, H.; Du, B.; Li, M.; Huang, X.; Zhou, Z.; Wang, J. Fabrication of silk microcapsules by layer-by-layer desolvation. Mater. Lett. 2018, 237, 109–112. [Google Scholar] [CrossRef]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Mathur, A.B.; Gupta, V. Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine 2010, 5, 807–820. [Google Scholar] [CrossRef]
- Pandey, V.; Haider, T.; Chandak, A.R.; Chakraborty, A.; Banerjee, S.; Soni, V. Surface modified silk fibroin nanoparticles for improved delivery of doxorubicin: Development, characterization, in-vitro studies. Int. J. Biol. Macromol. 2020, 164, 2018–2027. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Q.; Li, R.; Wang, J.; Zhen, X.; Yue, G.; Wang, H.; Cui, F.; Wu, F.; Yang, M.; et al. Facile Preparation of Paclitaxel Loaded Silk Fibroin Nanoparticles for Enhanced Antitumor Efficacy by Locoregional Drug Delivery. ACS Appl. Mater. Interfaces 2013, 5, 12638–12645. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Gil, A.L.; Pérez, S.A.; Cutillas, N.; Meyer, H.; Pedreño, M.; Aznar-Cervantes, S.D.; Janiak, C.; Cenis, J.L.; Ruiz, J. Antitumor properties of platinum(iv) prodrug-loaded silk fibroin nanoparticles. Dalton Trans. 2015, 44, 13513–13521. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein–polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin–procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef]
- Mohammadian, M.; Moghaddam, A.D.; Sharifan, A.; Dabaghi, P.; Hadi, S. Nanocomplexes of whey protein fibrillar aggregates and quercetin as novel multi-functional biopolymeric ingredients: Interaction, chemical structure, and bio-functionality. J. Iran. Chem. Soc. 2020, 17, 2481–2492. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H.; Cai, Y.; Martin, R.; Mangnolato, D. Traditional Herbal Medicines—The Role of Polyphenols. Planta Medica 1989, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Polyphenol–protein interactions. Biochem. J. 1974, 139, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Maldonado-Alvarez, L. Polyphenol-Protein Complexes and Their Consequences for the Redox Activity, Structure and Function of Honey. A Current View and New Hypothesis—A Review. Pol. J. Food Nutr. Sci. 2015, 65, 71–80. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Qin, D.; Bergenståhl, B. Influence of amphiphilic structures on the stability of polyphenols with different hydrophobicity. Sci. China Chem. 2007, 50, 121–126. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.-L.; Dai, F.-Y.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.-Z. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.; Malovaná, S.; Pérez, J.; Borges, T.; Montelongo, F.G. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The influence of the hydrophilic–lipophilic environment on the structure of silk fibroin protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Silva, M.; Castellanos, L.; Ottens, M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Central J. 2015, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Kerem, Z.; Bilkis, I.; Flaishman, M.A.; Sivan, L. Antioxidant Activity and Inhibition of α-Glucosidase by trans-Resveratrol, Piceid, and a Novel trans-Stilbene from the Roots of Israeli Rumex bucephalophorus L. J. Agric. Food Chem. 2006, 54, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef]
- Ajisawa, A. Dissolution of silk fibroin with calcium chloride/ethanol aqueous solution Studies on the dissolution of silk fibroin. (IX). J. Sericultural Sci. Jpn. 1998, 67, 91–94. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Anti-oxidant capacity of dietary polyphenols determined by ABTS assay: A kinetic expression of the results. Int. J. Food Sci. Technol. 2008, 43, 185–191. [Google Scholar] [CrossRef]












| The Concentration of Phenolic Compounds (mg/mL) | ||||
|---|---|---|---|---|
| Gallic Acid | Vanillic Acid | Trans-Resveratrol | Quercetin | |
| Experimental (HPLC) | 0.0548 | 0.0430 | 0.0768 | 0.1010 |
| Theoretical | 0.0583 | 0.0500 | 0.0783 | 0.1030 |
| Exp No | Independent Variables | Observed Response | |
|---|---|---|---|
| SF Concentration (mg/mL) | EtOH Concentration (%) | Average Particle Size (nm) | |
| 1 | 0.9 | 70 | 62.5 |
| 2 | 2 | 60 | 245 |
| 3 | 1.5 | 70 | 50 |
| 4 | 1.5 | 70 | 67.5 |
| 5 | 1.5 | 82 | 62.5 |
| 6 | 1 | 80 | 250 |
| 7 | 1.5 | 58 | 180 |
| 8 | 1 | 60 | 90 |
| 9 | 1.5 | 70 | 63 |
| 10 | 2 | 80 | 300 |
| 11 | 2.1 | 70 | 150 |
| 12 | 1.5 | 70 | 53 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 90,639.97 | 8 | 11,330.00 | 166.72 | 0.0007 |
| A-SF Concentration | 3828.13 | 1 | 3828.13 | 56.33 | 0.0049 |
| B-EtOH Concentration | 6903.13 | 1 | 6903.13 | 101.58 | 0.0021 |
| AB | 2756.25 | 1 | 2756.25 | 40.56 | 0.0078 |
| A² | 3055.38 | 1 | 3055.38 | 44.96 | 0.0068 |
| B² | 5270.18 | 1 | 5270.18 | 77.55 | 0.0031 |
| A²B | 17,663.44 | 1 | 17,663.44 | 259.92 | 0.0005 |
| AB² | 366.35 | 1 | 366.35 | 5.39 | 0.1029 |
| A²B² | 9597.11 | 1 | 9597.11 | 141.22 | 0.0013 |
| Pure Error | 203.87 | 3 | 67.96 | ||
| Cor Total | 90,843.84 | 11 |
| Exp No | Independent Variables | Observed Response | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Encapsulated Phenolic Compound Concentration (mg/mL) | |||||||||
| Std | Silk Fibroin Concentration (mg/mL) | EtOH Concentration (%) | Particle Size (nm) | Trans- Resveratrol | Quercetin | Gallic Acid | Vanillic Acid | Total Phenol Amount | Phenol mg/mg Silk |
| 1 | 1 | 60 | 150 | 1.79 | 1.22 | 0 | 0 | 3 | 0 |
| 2 | 2 | 60 | 50 | 1.58 | 1.22 | 0.23 | 2.2 | 5.22 | 2 |
| 3 | 1 | 80 | 190 | 1.49 | 0.615 | 0 | 1.9 | 4.01 | 2.67 |
| 4 | 2 | 80 | 100 | 1.82 | 1.012 | 0.13 | 2 | 4.96 | 1.66 |
| 5 | 0.9 | 70 | 130 | 1.54 | 0.92 | 0 | 0 | 2.45 | 1.82 |
| 6 | 2.1 | 70 | 150 | 2.13 | 1.22 | 0.23 | 0 | 3.57 | 1.13 |
| 7 | 1.5 | 58 | 250 | 1.83 | 2.12 | 3 × 10−2 | 0 | 3.97 | 1.77 |
| 8 | 1.5 | 82 | 150 | 1.63 | 0 | 3 × 10−2 | 2.2 | 3.86 | 1.72 |
| 9 | 1.5 | 70 | 200 | 1.91 | 1.02 | 0 | 3.1 | 6.03 | 2.68 |
| 10 | 1.5 | 70 | 203 | 1.94 | 1.22 | 0 | 2.8 | 5.95 | 2.65 |
| 11 | 1.5 | 70 | 201 | 1.92 | 1.82 | 0 | 2.9 | 6.64 | 2.95 |
| 12 | 1.5 | 70 | 204 | 1.93 | 1.12 | 0 | 2.9 | 5.95 | 2.64 |
| Response | Source | SS | DF | MS | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|---|
| Particle Size | Model | 32,675.67 | 8 | 4084.46 | 1225.34 | <0.0001 | 0.99 |
| A-SF Concentration | 200 | 1 | 200 | 60 | 0.0045 | ||
| B-EtOH Concentration | 5000 | 1 | 5000 | 1500 | <0.0001 | ||
| Pure Error | 10 | 3 | 3.33 | ||||
| Total | 32,685.67 | 11 | |||||
| Vanillic Acid Concentration | Model | 0.000 | 8 | 2.2 × 10−6 | 143.52 | 0.0009 | 0.99 |
| A-SF Concentration | 0.000 | 1 | 0.0000 | 2.84 | 1.0000 | ||
| B-EtOH Concentration | 2.4 × 10−6 | 1 | 2.4 × 10−6 | 432.32 | 0.0011 | ||
| Pure Error | 4.7 × 10−8 | 3 | 1.5 × 10−8 | ||||
| Total | 0 | 11 | |||||
| Trans-resveratrol Concentration | Model | 4.1 × 10−7 | 8 | 5.1 × 10−8 | 309.84 | 0.0003 | 0.99 |
| A-SF Concentration | 1.7 × 10−7 | 1 | 1.7 × 10−7 | 1044.30 | <0.0001 | ||
| B-EtOH Concentration | 2.0 × 10−8 | 1 | 2.0 × 10−8 | 120 | 0.0016 | ||
| Pure Error | 5.0 × 10−10 | 3 | 1.6 × 10−10 | ||||
| Total | 4.1 × 10−7 | 11 | |||||
| Quercetin Concentration | Model | 2.566 × 10−6 | 8 | 3.2 × 10−7 | 21.07 | 0.015 | 0.98 |
| A-SF Concentration | 4.5 × 10−8 | 1 | 4.5 × 10−8 | 0.35 | 0.18 | ||
| B-EtOH Concentration | 2.2 × 10−6 | 1 | 2.2 × 10−6 | 85.51 | 0.001 | ||
| Pure Error | 4.5 × 10−8 | 3 | 1.5 × 10−8 | ||||
| Total | 2.6 × 10−6 | 11 | |||||
| Gallic Acid Concentration | Model | 8.8 × 10−8 | 5 | 1.7 × 10−8 | 84.38 | <0.0001 | 0.98 |
| A-SF Concentration | 5.8 × 10−8 | 1 | 5.9 × 10−8 | 281.75 | <0.0001 | ||
| B-EtOH Concentration | 1.4 × 10−9 | 1 | 1.4 × 10−9 | 6.97 | 0.0386 | ||
| Pure Error | 0 | 3 | 0 | ||||
| Total | 8.9 × 10−8 | 11 |
| Exp No | Independent Variables | Observed Response | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Encapsulated Phenolic Compound Concentration | ||||||||||
| Std | Silk Fibroin Concentration (mg/mL) | EtOH Concentration (%) | pH | Particle Size (nm) | Gallic Acid (µg/mL) | Vanillic Acid (µg/mL) | Trans-Resveratrol (µg/mL) | Quercetin (µg/mL) | Total Phenol Amount (µg/mL) | Phenol mg/mg × 10−3 Silk |
| 1 | 1 | 60 | 2 | 250 | 0.8 | 0.3 | 1.7 | 1.01 | 3.81 | 3.81 |
| 2 | 2 | 60 | 2 | 303 | 0.3 | 0.3 | 1.7 | 0.91 | 3.21 | 1.07 |
| 3 | 1 | 80 | 2 | 402 | 0 | 0 | 2.1 | 0.91 | 3.01 | 2.01 |
| 4 | 2 | 80 | 2 | 302 | 0 | 0 | 1.4 | 0.71 | 2.11 | 0.70 |
| 5 | 1 | 60 | 7 | 120 | 0.2 | 0 | 2.4 | 1.21 | 3.81 | 2.54 |
| 6 | 2 | 60 | 7 | 120 | 0 | 0 | 2.3 | 1.01 | 3.31 | 1.10 |
| 7 | 1 | 80 | 7 | 380 | 0.1 | 0 | 1.6 | 1.01 | 2.71 | 1.81 |
| 8 | 2 | 80 | 7 | 303 | 0.4 | 0 | 2.4 | 1.21 | 4.01 | 1.34 |
| 9 | 0.9 | 70 | 4.5 | 302 | 0.2 | 0 | 1.6 | 0.71 | 2.51 | 1.86 |
| 10 | 2.1 | 70 | 4.5 | 500 | 0.1 | 0.2 | 1.9 | 0.81 | 3.01 | 0.96 |
| 11 | 1.5 | 58 | 4.5 | 550 | 0 | 0 | 1.7 | 0 | 1.7 | 0.76 |
| 12 | 1.5 | 82 | 4.5 | 303 | 0 | 0 | 1.1 | 0 | 1.1 | 0.49 |
| 13 | 1.5 | 70 | 1.5 | 500 | 0.2 | 0 | 1.8 | 1.11 | 3.11 | 1.38 |
| 14 | 1.5 | 70 | 7.5 | 500 | 0.2 | 0 | 1.2 | 0 | 1.4 | 0.62 |
| 15 | 1.5 | 70 | 4.5 | 403 | 0 | 0 | 1.6 | 0.7 | 2.3 | 1.02 |
| 16 | 1.5 | 70 | 4.5 | 405 | 0 | 0 | 1.6 | 0.7 | 2.3 | 1.02 |
| 17 | 1.5 | 70 | 4.5 | 402 | 0.1 | 0 | 1.7 | 0.91 | 2.71 | 1.20 |
| 18 | 1.5 | 70 | 4.5 | 377 | 0.1 | 0.1 | 1.7 | 0.91 | 2.81 | 1.25 |
| Response | Source | SS | DF | MS | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|---|
| Particle Size | Model | 2.4 × 105 | 14 | 17,575.31 | 100.48 | 0.0014 | 0.99 |
| A-SF Concentration | 19,602.00 | 1 | 19,602.00 | 112.06 | 0.0018 | ||
| B-EtOH Concentration | 30,504.50 | 1 | 30,504.50 | 174.39 | 0.0009 | ||
| pH | 0 | 1 | 0 | 0 | 0 | ||
| Pure Error | 524.75 | 3 | 174.92 | ||||
| Total | 2.466 × 105 | 17 | |||||
| Vanillic Acid Concentration | Model | 1.775 × 10−7 | 11 | 2.2 × 10−6 | 12.91 | 0.0026 | 0.95 |
| A-SF Concentration | 2.000 × 10−8 | 1 | 2.0 × 10−8 | 16.00 | 0.0071 | ||
| B-EtOH Concentration | 0.0000 | 1 | 0.0000 | 0.0000 | 1.000 | ||
| pH | 0.0000 | 1 | 0.0000 | 0.0000 | 1.000 | ||
| Pure Error | 7.500 × 10−9 | 3 | 2.5 × 10−9 | ||||
| Total | 1.850 × 10−7 | 17 | |||||
| Trans-resveratrol Concentration | Model | 2.2 × 10−6 | 14 | 1.5 × 10−7 | 47.89 | 0.0043 | 0.99 |
| A-SF Concentration | 4.5 × 10−8 | 1 | 4.5 × 10−8 | 13.50 | 0.0349 | ||
| B-EtOH Concentration | 1.8 × 10−7 | 1 | 1.8 × 10−7 | 54.00 | 0.0052 | ||
| pH | 1.8 × 10−7 | 1 | 1.8 × 10−7 | 1.50 | 0.0052 | ||
| Pure Error | 1.0 × 10−8 | 3 | 3.3 × 10−9 | ||||
| Total | 2.2 × 100 | 17 | |||||
| Quercetin Concentration | Model | 2.5 × 10−6 | 14 | 1.7 × 10−7 | 12.18 | 0.0313 | 0.98 |
| A-SF Concentration | 5.0 × 10−9 | 1 | 5.0 × 10−9 | 0.3401 | 0.6007 | ||
| B-EtOH Concentration | 0.0000 | 1 | 0.0000 | 0.0000 | 1.000 | ||
| pH | 6.1 × 10−7 | 1 | 6.1 × 10−7 | 41.91 | 0.0075 | ||
| Pure Error | 4.4 × 10−8 | 3 | 1.4 × 10−8 | ||||
| Total | 2.5 × 10−6 | 17 | |||||
| Gallic Acid Concentration | Model | 6.4 × 10−7 | 9 | 7.2 × 10−8 | 15.24 | 0.0004 | 0.94 |
| A-SF Concentration | 2.4 × 10−8 | 1 | 2.5 × 10−8 | 5.27 | 0.0509 | ||
| B-EtOH Concentration | 5.8 × 10−8 | 1 | 5.9 × 10−8 | 12.46 | 0.0077 | ||
| pH | 1.4 × 10−8 | 1 | 1.5 × 10−8 | 3.12 | 0.1155 | ||
| Pure Error | 1.0 × 10−8 | 3 | 3.3 × 10−9 | ||||
| Total | 6.8 × 107 | 17 |
| Phenol Compound | Initial Concentration (µg/mL) | Final Concentration (µg/mL) | Encapsulated Phenolic Compound (µg/mL) | Phenol (µg/mg 10−3) Silk Fibroin |
|---|---|---|---|---|
| Gallic Acid | 5.82 | 5.80 | 0 | 0 |
| Vanillic Acid | 3.4 | 3.4 | 0 | 0 |
| Trans-resveratrol | 07.03 | 5.1 | 1.93 | 0.65 |
| Quercetin | 10.3 | 10.0 | 1.31 | 0.44 |
| Sample\Rf Values | Unloaded Phenols | Loaded Phenols |
|---|---|---|
| PFmix | 0.3 and 0.19 | 0.32 and 0.19 |
| Trans-Resveratrol | 0.25 | 0.2 |
| Gallic Acid | 0.057 | 0.06 |
| Vanillic Acid | 0.34 | 0.32 |
| Quercetin | 0.23 | 0.13 |
| Control | - | - |
| Experimental Setup | Independent Variables | Unit | Factors’ Symbol | Coded Levels | |
|---|---|---|---|---|---|
| −1 | 1 | ||||
| Set 1 | Silk Fibroin Concentration | mg/ml | A | 1 | 2 |
| Set 2 | Ethanol concentration in Solution | % | B | 60 | 80 |
| Set 3 | pH | C | 2 | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayraktar, O.; Oder, G.; Erdem, C.; Kose, M.D.; Cheaburu-Yilmaz, C.N. Selective Encapsulation of the Polyphenols on Silk Fibroin Nanoparticles: Optimization Approaches. Int. J. Mol. Sci. 2023, 24, 9327. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24119327
Bayraktar O, Oder G, Erdem C, Kose MD, Cheaburu-Yilmaz CN. Selective Encapsulation of the Polyphenols on Silk Fibroin Nanoparticles: Optimization Approaches. International Journal of Molecular Sciences. 2023; 24(11):9327. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24119327
Chicago/Turabian StyleBayraktar, Oguz, Gizem Oder, Cansu Erdem, Merve Deniz Kose, and Catalina N. Cheaburu-Yilmaz. 2023. "Selective Encapsulation of the Polyphenols on Silk Fibroin Nanoparticles: Optimization Approaches" International Journal of Molecular Sciences 24, no. 11: 9327. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24119327