Recruitment of Corticotropin-Releasing Hormone (CRH) Neurons in Categorically Distinct Stress Reactions in the Mouse Brain
Abstract
:1. Introduction
2. Results
2.1. Validation
2.2. Distribution of tdTomato Positive Neurons in the Mouse Brain
2.3. Distribution of tdTomato Positive Fibers in the Mouse Brain
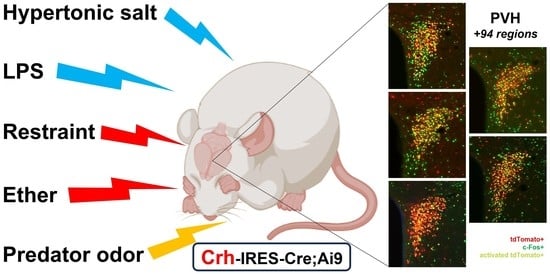
2.4. Neuronal Activation
2.4.1. Controls
2.4.2. Stress-Induced Neuronal Activation
2.4.3. Stress-Induced Activation of tdTomato Positive Profiles
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Acute Stress
4.2.1. Ether Inhalation
4.2.2. Hypertonic Salt
4.2.3. Restraint
4.2.4. Lipopolysaccharide Injection
4.2.5. Predator Odor
4.2.6. Controls
4.3. Adrenalectomy
4.4. Perfusion and Tissue Processing
4.5. Immunocytochemistry
4.6. RNAscope In Situ Hybridization (ISH)
4.7. Imaging, Quantification and Data Analysis
4.8. Corticosterone Measurement
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AId | Agranular insular area, dorsal part |
| CA | Ammon’s horn (Hippocampal Formation) |
| ACA | Anterior cingulate area |
| AHNc | Anterior hypothalamic nucleus |
| AHNp | Anterior hypothalamic nucleus, posterior part |
| AON | Anterior olfactory nucleus |
| ARH | Arcuate hypothalamic nucleus |
| AP | Area postrema |
| B | Barrington’s nucleus (Pontine Urinary Center) |
| BSTa | Bed nuclei of the stria terminalis, anterior division |
| BSTad | Bed nuclei of the stria terminalis, anterior division, dorsal part |
| BSTav | Bed nuclei of the stria terminalis, anterior division, ventral part |
| BSTp | Bed nuclei of the stria terminalis, posterior division |
| BAC | Bed nucleus of the anterior commissure |
| CEA | Central amygdalar nucleus |
| CL | Central lateral nucleus of the thalamus |
| CM | Central medial nucleus of the thalamus |
| CLA | Claustrum |
| CN | Cochlear nuclei |
| COA | Cortical amygdalar area |
| CTXsp | Cortical subplate |
| CU | Cuneate nucleus |
| DpMe | Deep mesencephalic nucleus |
| DG | Dentate gyrus |
| DMX | Dorsal motor nucleus of the vagus nerve |
| DP | Dorsal peduncular area |
| DTr | Dorsal transition zone |
| DMH | Dorsomedial nucleus of the hypothalamus |
| EW | Edinger-Westphal nucleus |
| Epd | Endopiriform nucleus, dorsal part |
| ECU | External cuneate nucleus |
| CA3 | Field CA3 |
| FS | Fundus of striatum |
| G | Geniculate nucleus |
| GU | Gustatory areas |
| XII | Hypoglossal nucleus |
| IO | Inferior olivary complex |
| ILA | Infralimbic area |
| IF | Interfascicular nucleus raphe |
| IPN | Interpeduncular nucleus |
| LVOV | Lateral and ventral orbital cortex ventral part |
| LHA | Lateral hypothalamic area |
| LRN | Lateral reticular nucleus |
| LS | Lateral septal nucleus |
| LC | Locus coeruleus |
| MCLHD | Magnocellular nucleus, lateral hypothalamic area dorsal part |
| MCLHV | Magnocellular nucleus, lateral hypothalamic area ventral part |
| MOBgl | Main olfactory bulb, glomerular layer |
| MOBopl | Main olfactory bulb, outer plexiform layer |
| MEA | Medial amygdalar nucleus |
| MPN | Medial preoptic nucleus |
| MV | Medial vestibular nucleus |
| RE | Nucleus of reuniens |
| NLOT | Nucleus of the lateral olfactory tract |
| NTS | Nucleus of the solitary tract |
| PRP | Nucleus prepositus |
| RPA | Nucleus prepositus |
| X | Nucleus x |
| Mm | Mus musculus |
| OT | Olfactory tubercle |
| O | Orbital cortex |
| PSTN | Parasubthalamic nucleus |
| PVH | Paraventricular hypothalamic nucleus |
| PVTa | Paraventricular nucleus of the thalamus, anterior |
| PVTp | Paraventricular nucleus of the thalamus, posterior |
| PAG | Periaqueductal gray |
| PeF | Perifornical nucleus |
| PVR | Periventricular region |
| PIR | Piriform area |
| PCG | Pontine central gray |
| PG | Pontine gray |
| PGdm | Pontine nucleus, dorsomedial part |
| PHd | Posterior hypothalamic nucleus, dorsal part |
| PHp | Posterior hypothalamic nucleus |
| PHv | Posterior hypothalamic nucleus, ventral part |
| PIL | Posterior intralaminar thalamic nucleus |
| PMTH | Posteromedial thalamic area [intermediodorsal nu of the thalamus; subparafascicular area] |
| PRC | Precommissural nucleus |
| PL | Prelimbic area |
| PM | Premammillary nucleus |
| Mop | Primary motor area |
| SSp | Primary somatosensory area |
| RSP | Retrosplenial area |
| RH | Rhomboid nucleus |
| MOs | Secondary motor area |
| SNc | Substantia nigra, compact part |
| CS | Superior central nucleus raphe |
| SCs | Superior colliculus, sensory related |
| SSs | Supplemental somatosensory area |
| SCH | Suprachiasmatic nucleus |
| SUM | Supramammillary nucleus |
| SO | Supraoptic nucleus |
| TTv | Taenia tecta, ventral part |
| TRN | Tegmental reticular nucleus |
| LGv | Ventral part of the lateral geniculate complex |
| VMH | Ventromedial hypothalamic nucleus |
| ZI | Zona incerta |
References
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Sawchenko, P.E.; Brown, E.R.; Chan, R.K.; Ericsson, A.; Li, H.Y.; Roland, B.L.; Kovacs, K.J. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog. Brain Res. 1996, 107, 201–222. [Google Scholar]
- Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology 1983, 36, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Berridge, C.W. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res. Brain Res. Rev. 1990, 15, 71–100. [Google Scholar] [CrossRef]
- Koob, G.F. A role for brain stress systems in addiction. Neuron 2008, 59, 11–34. [Google Scholar] [CrossRef] [Green Version]
- Linthorst, A.C.; Flachskamm, C.; Hopkins, S.J.; Hoadley, M.E.; Labeur, M.S.; Holsboer, F.; Reul, J.M. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J. Neurosci. 1997, 17, 4448–4460. [Google Scholar] [CrossRef] [Green Version]
- Devilbiss, D.M.; Waterhouse, B.D.; Berridge, C.W.; Valentino, R. Corticotropin-releasing factor acting at the locus coeruleus disrupts thalamic and cortical sensory-evoked responses. Neuropsychopharmacology 2012, 37, 2020–2030. [Google Scholar] [CrossRef] [Green Version]
- Kuperman, Y.; Weiss, M.; Dine, J.; Staikin, K.; Golani, O.; Ramot, A.; Nahum, T.; Kuhne, C.; Shemesh, Y.; Wurst, W.; et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell. Metab. 2016, 23, 1185–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchenthaler, I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides 1984, 5 (Suppl. S1), 53–69. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, E.J.; Colago, E.E.; Valentino, R.J. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: Substrate for the co-ordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol. 1998, 10, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Antoni, F.A.; Palkovits, M.; Makara, G.B.; Linton, E.A.; Lowry, P.J.; Kiss, J.Z. Immunoreactive corticotropin-releasing hormone in the hypothalamoinfundibular tract. Neuroendocrinology 1983, 36, 415–423. [Google Scholar] [CrossRef]
- Silverman, A.J. Modofication of hypothalamic neurons by behavioral stress. In Neuropeptides and Stress; Tache, I.M.J.E., Brown, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 23–37. [Google Scholar]
- Taniguchi, H.; He, M.; Wu, P.; Kim, S.; Paik, R.; Sugino, K.; Kvitsiani, D.; Fu, Y.; Lu, J.; Lin, Y.; et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 2011, 71, 995–1013. [Google Scholar] [CrossRef] [Green Version]
- Wamsteeker Cusulin, J.I.; Fuzesi, T.; Watts, A.G.; Bains, J.S. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS ONE 2013, 8, e64943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Long, B.; Yuan, J.; Peng, X.; Ni, H.; Li, X.; Gong, H.; Luo, Q.; Li, A. A Quantitative Analysis of the Distribution of CRH Neurons in Whole Mouse Brain. Front. Neuroanat. 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Molet, J.; Gunn, B.G.; Ressler, K.; Baram, T.Z. Diversity of Reporter Expression Patterns in Transgenic Mouse Lines Targeting Corticotropin-Releasing Hormone-Expressing Neurons. Endocrinology 2015, 156, 4769–4780. [Google Scholar] [CrossRef]
- Morgan, J.I.; Curran, T. Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 1991, 14, 421–451. [Google Scholar] [CrossRef]
- Kovacs, K.J. Measurement of immediate-early gene activation- c-fos and beyond. J. Neuroendocrinol. 2008, 20, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.K.; Brown, E.R.; Ericsson, A.; Kovacs, K.J.; Sawchenko, P.E. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J. Neurosci. 1993, 13, 5126–5138. [Google Scholar] [CrossRef]
- Park, A.Y.; Park, Y.S.; So, D.; Song, I.K.; Choi, J.E.; Kim, H.J.; Lee, K.J. Activity-Regulated Cytoskeleton-Associated Protein (Arc/Arg3.1) is Transiently Expressed after Heat Shock Stress and Suppresses Heat Shock Factor 1. Sci. Rep. 2019, 9, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawchenko, P.E.; Li, H.Y.; Ericsson, A. Circuits and mechanisms governing hypothalamic responses to stress: A tale of two paradigms. Prog. Brain Res. 2000, 122, 61–78. [Google Scholar] [PubMed]
- Rivest, S.; Rivier, C. Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: Comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J. Neuroendocrinol. 1994, 6, 101–117. [Google Scholar] [CrossRef]
- Radley, J.J.; Arias, C.M.; Sawchenko, P.E. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci. 2006, 26, 12967–12976. [Google Scholar] [CrossRef] [Green Version]
- Ericsson, A.; Kovacs, K.J.; Sawchenko, P.E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994, 14, 897–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayas, C.V.; Buller, K.M.; Crane, J.W.; Xu, Y.; Day, T.A. Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur. J. Neurosci. 2001, 14, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Cullinan, W.E.; Helmreich, D.L.; Watson, S.J. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 1996, 368, 88–99. [Google Scholar] [CrossRef]
- Campeau, S.; Watson, S.J. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997, 9, 577–588. [Google Scholar]
- Kier, A.; Han, J.; Jacobson, L. Chronic treatment with the monoamine oxidase inhibitor phenelzine increases hypothalamic-pituitary-adrenocortical activity in male C57BL/6 mice: Relevance to atypical depression. Endocrinology 2005, 146, 1338–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Nagano, M.; Suzuki, H.; Murakoshi, T. Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology 2010, 58, 746–757. [Google Scholar] [CrossRef]
- Niu, M.; Kasai, A.; Tanuma, M.; Seiriki, K.; Igarashi, H.; Kuwaki, T.; Nagayasu, K.; Miyaji, K.; Ueno, H.; Tanabe, W.; et al. Claustrum mediates bidirectional and reversible control of stress-induced anxiety responses. Sci. Adv. 2022, 8, eabi6375. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, J.Y.; Holmes, A.; Pan, B.X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Crestani, C.C.; Alves, F.H.; Gomes, F.V.; Resstel, L.B.; Correa, F.M.; Herman, J.P. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: A review. Curr. Neuropharmacol. 2013, 11, 141–159. [Google Scholar] [CrossRef] [Green Version]
- Campeau, S.; Akil, H.; Watson, S.J. Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mRNA in the forebrain associated with audiogenic stress in rats. J. Neurosci. 1997, 17, 5979–5992. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Zhao, J.; Rahman, M.; St-Cyr, S.; McGowan, P.O.; Kim, J.C. Hippocampus-Anterior Hypothalamic Circuit Modulates Stress-Induced Endocrine and Behavioral Response. Front. Neural Circuits 2022, 16, 894722. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Costa-Ferreira, W.; Mendonca, M.M.; Xavier, C.H.; Crestani, C.C. Lateral hypothalamus involvement in control of stress response by bed nucleus of the stria terminalis endocannabinoid neurotransmission in male rats. Sci. Rep. 2021, 11, 16133. [Google Scholar] [CrossRef] [PubMed]
- DiMicco, J.A.; Samuels, B.C.; Zaretskaia, M.V.; Zaretsky, D.V. The dorsomedial hypothalamus and the response to stress: Part renaissance, part revolution. Pharm. Pharmacol. Biochem. Behav. 2002, 71, 469–480. [Google Scholar] [CrossRef]
- Myers, B.; Carvalho-Netto, E.; Wick-Carlson, D.; Wu, C.; Naser, S.; Solomon, M.B.; Ulrich-Lai, Y.M.; Herman, J.P. GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity. Neuropsychopharmacology 2016, 41, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, T.; Tohyama, S.; Mikami, K.; Nagase, M.; Morishima, M.; Kasai, A.; Hashimoto, H.; Watabe, A.M. Parabrachial-to-parasubthalamic nucleus pathway mediates fear-induced suppression of feeding in male mice. Nat. Commun. 2022, 13, 7913. [Google Scholar] [CrossRef] [PubMed]
- McGirr, A.; LeDue, J.; Chan, A.W.; Boyd, J.D.; Metzak, P.D.; Murphy, T.H. Stress impacts sensory variability through cortical sensory activity motifs. Transl. Psychiatry 2020, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.T.; Lombardo, K.A.; Bakshi, V.P.; Balachandran, J.S.; Roseboom, P.H.; Kalin, N.H. Acute stress-induced increases in thalamic CRH mRNA are blocked by repeated stress exposure. Brain Res. 2001, 915, 18–24. [Google Scholar] [CrossRef]
- Hsu, D.T.; Lombardo, K.A.; Herringa, R.J.; Bakshi, V.P.; Roseboom, P.H.; Kalin, N.H. Corticotropin-releasing hormone messenger RNA distribution and stress-induced activation in the thalamus. Neuroscience 2001, 105, 911–921. [Google Scholar] [CrossRef]
- Tortorella, S.; Rodrigo-Angulo, M.L.; Nunez, A.; Garzon, M. Synaptic interactions between perifornical lateral hypothalamic area, locus coeruleus nucleus and the oral pontine reticular nucleus are implicated in the stage succession during sleep-wakefulness cycle. Front. Neurosci. 2013, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, A.; Brody, N.; Reddi, P.; Guo, J.; Gordon Rainnie, D.; Dias, B.G. Modulation of fear generalization by the zona incerta. Proc. Natl. Acad. Sci. USA 2019, 116, 9072–9077. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, P.; Qi, G.; Cai, H.; Li, T.; Li, M.; Cui, C.; Lei, J.; Ren, K.; Yang, J.; et al. A zona incerta-basomedial amygdala circuit modulates aversive expectation in emotional stress-induced aversive learning deficits. Front. Cell. Neurosci. 2022, 16, 910699. [Google Scholar] [CrossRef]
- Zhou, H.; Xiang, W.; Huang, M. Inactivation of Zona Incerta Blocks Social Conditioned Place Aversion and Modulates Post-traumatic Stress Disorder-Like Behaviors in Mice. Front. Behav. Neurosci. 2021, 15, 743484. [Google Scholar] [CrossRef]
- Romero-Leguizamon, C.R.; Kohlmeier, K.A. Stress-related endogenous neuropeptides induce neuronal excitation in the Laterodorsal Tegmentum. Eur. Neuropsychopharmacol. 2020, 38, 86–97. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.C.; Cornish, L.C.; Lawrence, A.J.; Campbell, E.J. The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: A validation study. Neuropharmacology 2018, 154, 96–106. [Google Scholar] [CrossRef]
- Kovacs, K.J.; Sawchenko, P.E. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J. Neurosci. 1996, 16, 262–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, I.M.; Kovacs, K.J. Postnatal handling alters the activation of stress-related neuronal circuitries. Eur. J. Neurosci. 2000, 12, 3003–3014. [Google Scholar] [CrossRef]
- Li, H.Y.; Sawchenko, P.E. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: A comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J. Comp. Neurol. 1998, 393, 244–266. [Google Scholar] [CrossRef]
- Pacak, K.; Palkovits, M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr. Rev. 2001, 22, 502–548. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Ericsson, A.; Sawchenko, P.E. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc. Natl. Acad. Sci. USA 1996, 93, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C.; Koob, G.F. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J. Pharm. Pharmacol. Exp. Ther. 2004, 311, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Servatius, R.J.; Beck, K.D.; Moldow, R.L.; Salameh, G.; Tumminello, T.P.; Short, K.R. A stress-induced anxious state in male rats: Corticotropin-releasing hormone induces persistent changes in associative learning and startle reactivity. Biol. Psychiatry 2005, 57, 865–872. [Google Scholar] [CrossRef]
- Roozendaal, B.; Schelling, G.; McGaugh, J.L. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. J. Neurosci. 2008, 28, 6642–6651. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, M.K.; Gourley, S.L. The stressed orbitofrontal cortex. Behav. Neurosci. 2021, 135, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Shima, Y.; Nakajima, K.; Nakamura, K. A central master driver of psychosocial stress responses in the rat. Science 2020, 367, 1105–1112. [Google Scholar] [CrossRef]
- Korte, S.M.; Bouws, G.A.; Bohus, B. Central actions of corticotropin-releasing hormone (CRH) on behavioral, neuroendocrine, and cardiovascular regulation: Brain corticoid receptor involvement. Horm. Behav. 1993, 27, 167–183. [Google Scholar] [CrossRef]
- Elsaafien, K.; Kirchner, M.K.; Mohammed, M.; Eikenberry, S.A.; West, C.; Scott, K.A.; de Kloet, A.D.; Stern, J.E.; Krause, E.G. Identification of Novel Cross-Talk between the Neuroendocrine and Autonomic Stress Axes Controlling Blood Pressure. J. Neurosci. 2021, 41, 4641–4657. [Google Scholar] [CrossRef]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Bruzsik, B.; Biro, L.; Sarosdi, K.R.; Zelena, D.; Sipos, E.; Szebik, H.; Torok, B.; Mikics, E.; Toth, M. Neurochemically distinct populations of the bed nucleus of stria terminalis modulate innate fear response to weak threat evoked by predator odor stimuli. Neurobiol. Stress. 2021, 15, 100415. [Google Scholar] [CrossRef] [PubMed]
- Kasckow, J.W.; Baker, D.; Geracioti, T.D., Jr. Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides 2001, 22, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.L.; Young, W.S., 3rd. Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J. Physiol. 1988, 403, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Isosaka, T.; Tang, L.; Soga, T.; Kobayakawa, R.; Kobayakawa, K. Artificial hibernation/life-protective state induced by thiazoline-related innate fear odors. Commun. Biol. 2021, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Zelena, D.; Mergl, Z.; Foldes, A.; Kovacs, K.J.; Toth, Z.; Makara, G.B. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1110–E1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Target | Catalog Number | Fluorophores | Fluorophore Dilution |
|---|---|---|---|
| Mm *-Crh | 316091 | Fluorescein Plus TSA | 1:3000 |
| 3-plex Positive Control Probe-Mm | 320881 | Fluorescein Plus TSA | 1:3000 |
| 3-plex Negative Control Probe | 320871 | Fluorescein Plus TSA | 1:3000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, K.; Juhász, B.; Kuti, D.; Ferenczi, S.; Kovács, K.J. Recruitment of Corticotropin-Releasing Hormone (CRH) Neurons in Categorically Distinct Stress Reactions in the Mouse Brain. Int. J. Mol. Sci. 2023, 24, 11736. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms241411736
Horváth K, Juhász B, Kuti D, Ferenczi S, Kovács KJ. Recruitment of Corticotropin-Releasing Hormone (CRH) Neurons in Categorically Distinct Stress Reactions in the Mouse Brain. International Journal of Molecular Sciences. 2023; 24(14):11736. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms241411736
Chicago/Turabian StyleHorváth, Krisztina, Balázs Juhász, Dániel Kuti, Szilamér Ferenczi, and Krisztina J. Kovács. 2023. "Recruitment of Corticotropin-Releasing Hormone (CRH) Neurons in Categorically Distinct Stress Reactions in the Mouse Brain" International Journal of Molecular Sciences 24, no. 14: 11736. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms241411736