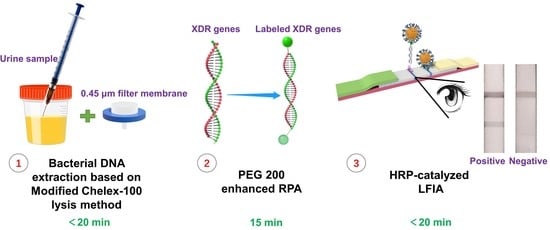
MC-PRPA-HLFIA Cascade Detection System for Point-of-Care Testing Pan-Drug-Resistant Genes in Urinary Tract Infection Samples
Abstract
:1. Introduction
2. Results
2.1. Principles
2.1.1. Nfo-RPA
2.1.2. LFIA Catalyzed by HRP
2.2. Within 20 min, a Modified Chelex-100 Lysis Technique Could Recover DNA from Urine Containing Uncultured Bacteria
2.3. Successful Fabrication of AuNPs and HRP-AuNPs-Antibody Conjugates
2.4. Optimization of the Detection Conditions
2.4.1. PEG 200 Enhanced RPA
2.4.2. HRP-Catalyzed LFIA
2.5. The Cascade System’s Sensitivity for Detecting Pan-Drug-Resistant Genes in Urine Samples Reached 102 CFU/mL
2.6. The Cascade Detection System Has Favorable Specificity
2.7. Evaluation of Cascade Detection Method Sensitivity and Specificity in Identifying the Pan-Drug-Resistant Genes in Simulated and Real UTI Samples
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Construction of Standard Bacterial Strains
4.3. Preparation of Simulated UTI Samples
4.4. Bacterial DNA Extraction from the Bacteria-Containing Urine Sample
- To prepare the Chelex-100 lysis solution, combine 2.5 g Chelex-100, 50 mL TE buffer, and 500 µL TritonX-100 in a container and thoroughly mix.
- Add 200 µL of Chelex-100 lysis solution to the prepared bacterial-containing urine sample and mix completely.
- Heat the mixture at 100 °C for 10 min.
- A 1 mL syringe is used to aspirate the mixture, which is then filtered by a 0.45 µm filter membrane.
4.5. PEG 200 Enhanced RPA
4.5.1. Primers and Probes Design for RPA
4.5.2. Enhanced RPA by PEG 200
4.6. HRP-Catalyzed Lateral Flow Immunoassay
4.6.1. Synthesis of HRP-AuNPs-Antibody Conjugate
4.6.2. Manufacture of the Strip
4.7. Cascade System for Detection of Pan-Drug-Resistant Gene in Uncultured Urine Samples
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Nesi, G.; Mazzoli, S.; Meacci, F.; Lanzafame, P.; Caciagli, P.; Mereu, L.; Tateo, S.; Malossini, G.; Selli, C.; et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin. Infect. Dis. 2015, 61, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.F.; D’Souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug. Resist. Update 2016, 29, 30–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhai, W.; Song, H.; Fu, Y.; Schwarz, S.; He, T.; Bai, L.; Wang, Y.; Walsh, T.R.; Shen, J. Identification of the novel tigecycline resistance gene tet(X6) and its variants in Myroides, Acinetobacter and Proteus of food animal origin. J. Antimicrob. Chemother. 2020, 75, 1428–1431. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: Report from the China CRE network. Antimicrob. Agents. Chemother. 2018, 62, e01882-17. [Google Scholar] [CrossRef] [Green Version]
- Ming, D.S.; Chen, Q.Q.; Chen, X.T. Analysis of resistance genes in pan-resistant Myroides odoratimimus clinical strain PR63039 using whole genome sequencing. Microb. Pathog. 2017, 112, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ara, B.; Urmi, U.L.; Haque, T.A.; Nahar, S.; Rumnaz, A.; Ali, T.; Alam, M.S.; Mosaddek, A.; Rahman, N.; Haque, M.; et al. Detection of mobile colistin-resistance gene variants (mcr-1 and mcr-2) in urinary tract pathogens in Bangladesh: The last resort of infectious disease management colistin efficacy is under threat. Expert. Rev. Clin. Pharmacol. 2021, 14, 513–522. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Yan, H.; Zhu, Y.; Wang, L.; Zhang, Y.; Lu, Y.; Xing, W. Sensitive and rapid detection of pathogenic bacteria from urine samples using multiplex recombinase polymerase amplification. Lab Chip 2018, 18, 2441–2452. [Google Scholar] [CrossRef]
- Hu, C.; Kalsi, S.; Zeimpekis, I.; Sun, K.; Ashburn, P.; Turner, C.; Sutton, J.M.; Morgan, H. Ultra-fast electronic detection of antimicrobial resistance genes using isothermal amplification and Thin Film Transistor sensors. Biosens. Bioelectron. 2017, 96, 281–287. [Google Scholar] [CrossRef]
- Choi, G.; Jung, J.H.; Park, B.H.; Oh, S.J.; Seo, J.H.; Choi, J.S.; Kim, D.H.; Seo, T.S. A centrifugal direct recombinase polymerase amplification (direct-RPA) microdevice for multiplex and real-time identification of food poisoning bacteria. Lab Chip 2016, 16, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Liu, D.; Xiong, J.; Dou, L.; Zhai, W.; Zhang, R.; Wang, Y.; Shen, J.; Wen, K. Rapid on-site detection of extensively drug-resistant genes in Enterobacteriaceae via enhanced recombinase polymerase amplification and lateral flow biosensor. Microbiol. Spectr. 2022, 10, e0334422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Shang, Y.; Xu, Y.; Zhang, L.; Luo, Y.; Huang, K.; Xu, W. On-site detection of stacked genetically modified soybean based on event-specific TM-LAMP and a DNAzyme-lateral flow biosensor. Biosens. Bioelectron. 2017, 91, 408–416. [Google Scholar] [CrossRef]
- Li, X.; Mu, X.; Zhang, P.; Zhao, D.; Ji, J.; Quan, J.; Zhu, Y.; Yu, Y. Detection and characterization of a clinical Escherichia coli ST3204 strain coproducing NDM-16 and MCR-1. Infect. Drug Resist. 2018, 11, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Dortet, L.; Cotellon, G.; Creton, E.; Cuzon, G.; Ponties, V.; Bonnin, R.A.; Naas, T. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob. Agents Chemother. 2018, 62, e00266-18. [Google Scholar] [CrossRef] [Green Version]
- Norian, H.; Field, R.M.; Kymissis, I.; Shepard, K.L. An integrated CMOS quantitative-polymerase-chain-reaction lab-on-chip for point-of-care diagnostics. Lab Chip 2014, 14, 4076–4084. [Google Scholar] [CrossRef]
- Kulinski, M.D.; Mahalanabis, M.; Gillers, S.; Zhang, J.Y.; Singh, S.; Klapperich, C.M. Sample preparation module for bacterial lysis and isolation of DNA from human urine. Biomed. Microdevices 2009, 11, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Liu, W.; Zhang, J.; Wang, S.; Yang, F.; Fang, Y.; Jiang, W.; Ding, L.; Zhao, H.; Zhang, Y. The direct semi-quantitative detection of 18 pathogens and simultaneous screening for nine resistance genes in clinical urine samples by a high-throughput multiplex genetic detection system. Front. Cell. Infect. Microbiol. 2021, 11, 660461. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, W.; Zhang, S.; Li, Q.; Wang, Y.; Chen, T.; Jiang, H.; Kong, D.; Lv, Q.; Zheng, Y.; et al. Rapid detection of bacterial pathogens and antimicrobial resistance genes in clinical urine samples with urinary tract infection by metagenomic nanopore sequencing. Front. Microbiol. 2022, 13, 858777. [Google Scholar] [CrossRef]
- Jakobsen, L.; Garneau, P.; Kurbasic, A.; Bruant, G.; Stegger, M.; Harel, J.; Jensen, K.S.; Brousseau, R.; Hammerum, A.M.; Frimodt-Moller, N. Microarray-based detection of extended virulence and antimicrobial resistance gene profiles in phylogroup B2 Escherichia coli of human, meat and animal origin. J. Med. Microbiol. 2011, 60, 1502–1511. [Google Scholar] [CrossRef]
- Peter, H.; Berggrav, K.; Thomas, P.; Pfeifer, Y.; Witte, W.; Templeton, K.; Bachmann, T.T. Direct detection and genotyping of Klebsiella pneumoniae carbapenemases from urine by use of a new DNA microarray test. J. Clin. Microbiol. 2012, 50, 3990–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oviano, M.; Ramirez, C.L.; Barbeyto, L.P.; Bou, G. Rapid direct detection of carbapenemase-producing Enterobacteriaceae in clinical urine samples by MALDI-TOF MS analysis. J. Antimicrob. Chemother. 2017, 72, 1350–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Yang, J.S.; Kim, Y.; Kim, K.; Choi, H.; Lee, S.H. Comparison of DNA extraction methods for drug susceptibility testing by allele-specific primer extension on a microsphere-based platform: Chelex-100 (in-house and commercialized) and MagPurix TB DNA Extraction Kit. J. Microbiol. Methods 2018, 152, 105–108. [Google Scholar] [CrossRef]
- Higgins, M.; Ravenhall, M.; Ward, D.; Phelan, J.; Ibrahim, A.; Forrest, M.S.; Clark, T.G.; Campino, S. PrimedRPA: Primer design for recombinase polymerase amplification assays. Bioinformatics 2019, 35, 682–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Cheng, N.; Xu, Y.; Huang, K.; Luo, Y.; Xu, W. Point-of-care and visual detection of P. aeruginosa and its toxin genes by multiple LAMP and lateral flow nucleic acid biosensor. Biosens. Bioelectron. 2016, 81, 317–323. [Google Scholar] [CrossRef]
- Xu, W.; Cheng, N.; Huang, K.; Lin, Y.; Wang, C.; Xu, Y.; Zhu, L.; Du, D.; Luo, Y. Accurate and easy-to-use assessment of contiguous DNA methylation sites based on proportion competitive quantitative-PCR and lateral flow nucleic acid biosensor. Biosens. Bioelectron. 2016, 80, 654–660. [Google Scholar] [CrossRef] [PubMed]





| Pan-Drug Resistant Genes | Actually (+), MCL-PRPA-HLFIA (+) | Actually (+), MCL-PRPA-HLFIA (−) | Actually (−), MCL-PRPA-HLFIA (+) | Actually (−), MCL-PRPA-HLFIA (−) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| mcr-1 | 24 | 1 | 1 | 69 | 96.0 (24/24 + 1) | 98.6 (69/69 + 1) |
| blaNDM | 19 | 1 | 2 | 73 | 95.0 (19/19 + 1) | 97.3 (73/73 + 2) |
| blaKPC | 21 | 1 | 2 | 71 | 95.5 (21/21 + 1) | 97.3 (71/71 + 2) |
| tet(X) | 23 | 1 | 2 | 69 | 95.8 (23/23 + 1) | 97.2 (69/69 + 2) |
| Pan-Drug Resistant Genes | GCM (+), MCL-PRPA-HLFIA (+) | GCM (+), MCL-PRPA-HLFIA (−) | GCM (−), MCL-PRPA-HLFIA (+) | GCM (−), MCL-PRPA-HLFIA (−) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| mcr-1 | 1 | 0 | 0 | 31 | 100.0 (1/0 + 1) | 100.0 (31/0 + 31) |
| blaNDM | 0 | 0 | 0 | 32 | - | 100.0 (32/0 + 32) |
| blaKPC | 1 | 0 | 1 | 30 | 100.0 (1/0 + 1) | 96.8 (30/1 + 30) |
| tet(X) | 0 | 0 | 1 | 31 | - | 96.9 (31/1 + 31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Liu, D.; Xiong, J.; Shan, W.; Dou, L.; Zhai, W.; Wang, Y.; Shen, J.; Wen, K. MC-PRPA-HLFIA Cascade Detection System for Point-of-Care Testing Pan-Drug-Resistant Genes in Urinary Tract Infection Samples. Int. J. Mol. Sci. 2023, 24, 6784. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24076784
Tao J, Liu D, Xiong J, Shan W, Dou L, Zhai W, Wang Y, Shen J, Wen K. MC-PRPA-HLFIA Cascade Detection System for Point-of-Care Testing Pan-Drug-Resistant Genes in Urinary Tract Infection Samples. International Journal of Molecular Sciences. 2023; 24(7):6784. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24076784
Chicago/Turabian StyleTao, Jin, Dejun Liu, Jincheng Xiong, Wenchong Shan, Leina Dou, Weishuai Zhai, Yang Wang, Jianzhong Shen, and Kai Wen. 2023. "MC-PRPA-HLFIA Cascade Detection System for Point-of-Care Testing Pan-Drug-Resistant Genes in Urinary Tract Infection Samples" International Journal of Molecular Sciences 24, no. 7: 6784. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms24076784