Aliskiren-Loaded Nanoparticles Downregulate (Pro)renin Receptor and ACE Gene Expression in the Heart of Spontaneously Hypertensive Rats: Effect on NADPH Oxidase
Abstract
:1. Introduction
2. Results
2.1. Weight Parameters
2.2. Distribution of Polymeric Nanoparticles in the Heart
2.3. Quantitative Analysis of Aliskiren in the Heart
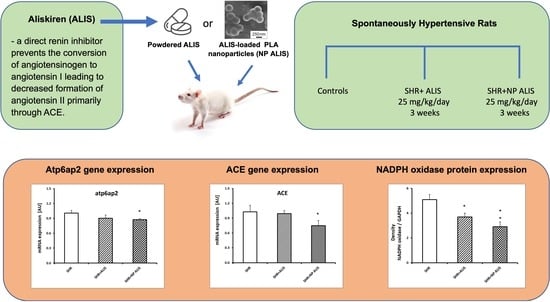
2.4. Profiling of Gene Expression
2.5. Protein Expression of NADPH Oxidase and Concentration of Conjugated Diene
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Polymeric Nanoparticles: Preparation and Characterization
4.3. Animals and Treatment
4.4. Distribution of Polymeric Nanoparticles in the Heart
4.5. Quantitative Analysis of Aliskiren in the Heart
4.6. Profiling of Gene Expression
4.7. Protein Expression of NADPH Oxidase and Concentration of Conjugated Diene
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bomback, A.S.; Toto, R. Dual blockade of the renin-angiotensin-aldosterone system: Beyond the ACE inhibitor and angiotensin-II receptor blocker combination. Am. J. Hypertens. 2009, 22, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Fraune, C.; Lange, S.; Krebs, C.; Hölzel, A.; Baucke, J.; Divac, N.; Schwedhelm, E.; Streichert, T.; Velden, J.; Garrelds, I.M.; et al. AT1 antagonism and renin inhibition in mice: Pivotal role of targeting angiotensin II in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2012, 303, F1037–F1048. [Google Scholar] [CrossRef]
- Chen, L.; Kim, S.M.; Eisner, C.; Oppermann, M.; Huang, Y.; Mizel, D.; Li, L.; Chen, M.; Lopez, M.L.S.; Weinstein, L.S.; et al. Stimulation of renin secretion by angiotensin II blockade is Gsalpha-dependent. J. Am. Soc. Nephrol. 2010, 21, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Abassi, Z.; Winaver, J.; Feuerstein, G.Z. The biochemical pharmacology of renin inhibitors: Implications for translational medicine in hypertension, diabetic nephropathy and heart failure: Expectations and reality. Biochem. Pharmacol. 2009, 78, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Maibaum, J.; Rahuel, J.; Grütter, M.G.; Cohen, N.C.; Rasetti, V.; Rüger, H.; Göschke, R.; Stutz, S.; Fuhrer, W.; et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem. Biophys. Res. Commun. 2003, 308, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Pantzaris, N.D.; Karanikolas, E.; Tsiotsios, K.; Velissaris, D. Renin inhibition with aliskiren: A decade of clinical experience. J. Clin. Med. 2017, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Rahuel, J.; Rasetti, V.; Maibaum, J.; Rüeger, H.; Göschke, R.; Cohen, N.C.; Stutz, S.; Cumin, F.; Fuhrer, W.; Wood, J.M.; et al. Structure-based drug design: The discovery of novel nonpeptide orally active inhibitors of human renin. Chem. Biol. 2000, 7, 493–504. [Google Scholar] [CrossRef]
- Lizakowski, S.; Tylicki, L.; Rutkowski, B. Direct renin inhibition—A promising strategy for renal protection? Med. Sci. Monit. 2013, 19, 451–457. [Google Scholar]
- Oparil, S. Role of aliskiren in cardio-renal protection and use in hypertensives with multiple risk factors. Vasc. Health Risk Manag. 2009, 5, 453. [Google Scholar] [CrossRef]
- Khan, V.; Hassan, M.Q.; Akhtar, M.; Najmi, A.K. Renin inhibition by aliskiren protects rats against isoproterenol induced myocardial infarction. Drug Res. 2018, 68, 139–145. [Google Scholar] [CrossRef]
- Ferri, N.; Greco, C.M.; Maiocchi, G.; Corsini, A. Aliskiren reduces prorenin receptor expression and activity in cultured human aortic smooth muscle cells. J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Biswas, K.B.; Nabi, A.H.M.N.; Nakagawa, T.; Suzuki, F.; Ebihara, A. Aliskiren reduces the release of soluble (pro)renin receptor from human umbilical vein endothelial cells. Biomed. Rep. 2018, 9, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Yong, Q.C.; Seqqat, R.; Chandel, N.; Feldman, D.L.; Baker, K.M.; Kumar, R. Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: Comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor. Clin. Sci. 2013, 124, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.A.; Advani, A.; Kim, S.; Advani, S.L.; Zhang, M.; White, K.E.; Kim, Y.M.; Parker, C.; Thai, K.; Krum, H.; et al. The cardiac (pro)renin receptor is primarily expressed in myocyte transverse tubules and is increased in experimental diabetic cardiomyopathy. J. Hypertens. 2011, 29, 1175–1184. [Google Scholar] [CrossRef]
- Nabi, A.H.M.N.; Biswas, K.B.; Ebihara, A.; Nakagawa, T.; Suzuki, F. Renin angiotensin system in the context of renin, prorenin, and the (pro)renin receptor. Rev. Agric. Sci. 2013, 1, 43–60. [Google Scholar] [CrossRef]
- Lian, H.; Wang, X.; Wang, J.; Liu, N.; Zhang, L.; Lu, Y.; Yang, Y.; Zhang, L. Heart-specific overexpression of (pro)renin receptor induces atrial fibrillation in mice. Int. J. Cardiol. 2015, 184, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Takemitsu, T.; Ichihara, A.; Kaneshiro, Y.; Sakoda, M.; Kurauchi-Mito, A.; Narita, T.; Kinouchi, K.; Yamashita, N.; Itoh, H. Association of (pro)renin receptor mRNA expression with angiotensin-converting enzyme mRNA expression in human artery. Am. J. Nephrol. 2009, 30, 361–370. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Chan, C.C.; Lee, K.C.; Huang, Y.T.; Lee, F.Y.; Yang, Y.Y.; Lin, H.C. Aliskiren reduces portal pressure and intrahepatic resistance in biliary cirrhotic rats. J. Chin. Med. Assoc. 2012, 75, 501–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Song, Y.; Zhao, X.; Wong, M.S.; Zhang, W. Renin inhibitor aliskiren exerts beneficial effect on trabecular bone by regulating skeletal renin-angiotensin system and kallikrein-kinin system in ovariectomized mice. Osteoporos. Int. 2016, 27, 1083–1092. [Google Scholar] [CrossRef]
- Pöss, J.; Werner, C.; Lorenz, D.; Gensch, C.; Böhm, M.; Laufs, U. The renin inhibitor aliskiren upregulates pro-angiogenic cells and reduces atherogenesis in mice. Basic Res. Cardiol. 2010, 105, 725–735. [Google Scholar] [CrossRef]
- Wang, L.P.; Fan, S.J.; Li, S.M.; Wang, X.J.; Sun, N. Aliskiren inhibits proliferation of cardiac fibroblasts in AGT-REN double transgenic hypertensive mice in vitro. Sheng Li Xue Bao 2016, 68, 684–690. [Google Scholar] [PubMed]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Wuerzner, G.; Azizi, M. Renin inhibition with aliskiren. Clin. Exp. Pharmacol. Physiol. 2008, 35, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.A.; Fortin, P.M.; Bassett, K.; Wright, J.M.; Musini, V.M. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst. Rev. 2017, 2017, CD007066. [Google Scholar]
- Ohara, M.; Ohyama, Y. Delivery and application of dietary polyphenols to target organs, tissues and intracellular organelles. Curr. Drug Metab. 2014, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Dayar, E.; Cebova, M. Therapeutic Potential of Polyphenols-Loaded Polymeric Nanoparticles in Cardiovascular System. Molecules 2020, 25, 3322. [Google Scholar] [CrossRef]
- Nguyen, G.; Delarue, F.; Burcklé, C.; Bouzhir, L.; Giller, T.; Sraer, J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Investig. 2002, 109, 1417–1427. [Google Scholar] [CrossRef]
- Moilanen, A.M.; Rysä, J.; Serpi, R.; Mustonen, E.; Szabò, Z.; Aro, J.; Näpänkangas, J.; Tenhunen, O.; Sutinen, M.; Salo, T.; et al. (Pro)renin receptor triggers distinct angiotensin II-independent extracellular matrix remodeling and deterioration of cardiac function. PLoS ONE 2012, 7, e41404. [Google Scholar] [CrossRef]
- Hennrikus, M.; Gonzalez, A.A.; Prieto, M.C. The prorenin receptor in the cardiovascular system and beyond. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H139–H145. [Google Scholar] [CrossRef]
- Fisher, N.D.; Hollenberg, N.K. Renin inhibition: What are the therapeutic opportunities? J. Am. Soc. Nephrol. 2005, 16, 592–599. [Google Scholar] [CrossRef]
- Giani, J.F.; Veiras, L.C.; Shen, J.Z.; Bernstein, E.A.; Cao, D.; Okwan-Duodu, D.; Khan, Z.; Gonzalez-Villalobos, R.A.; Bernstein, K.E. Novel roles of the renal angiotensin-converting enzyme. Mol. Cell. Endocrinol. 2021, 529, 111257. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Barta, A.; Koneracka, M.; Zavisova, V.; Kubovcikova, M.; Klimentova, J.; Török, J.; Zemancikova, A.; Cebova, M. Protective Effects of nanoparticle-loaded aliskiren on cardiovascular system in spontaneously hypertensive rats. Molecules 2019, 24, 2710. [Google Scholar] [CrossRef]
- Cao, X.; Yu, S.; Wang, Y.; Yang, M.; Xiong, J.; Yuan, H.; Dong, B. Effects of the (pro)renin receptor on cardiac remodeling and function in a rat alcoholic cardiomyopathy model via the PRR-ERK1/2-NOX4 pathway. Oxidative Med. Cell. Longev. 2019, 2019, 4546975. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kinugawa, S.; Takada, S.; Matsumoto, J.; Furihata, T.; Mizushima, W.; Tsuda, M.; Yokota, T.; Matsushima, S.; Okita, K.; et al. Direct renin inhibitor ameliorates insulin resistance by improving insulin signaling and oxidative stress in the skeletal muscle from post-infarct heart failure in mice. Eur. J. Pharmacol. 2016, 779, 147–156. [Google Scholar] [CrossRef]
- Peng, H.; Li, W.; Seth, D.M.; Nair, A.R.; Francis, J.; Feng, Y. (Pro)renin receptor mediates both angiotensin II-dependent and -independent oxidative stress in neuronal cells. PLoS ONE 2013, 8, e58339. [Google Scholar] [CrossRef] [PubMed]
- Rashikh, A.; Abul, K.N.; Akhtar, M.; Mahmood, D.; Pillai, K.K.; Ahmad, S.J. Protective effects of aliskiren in doxorubicin-induced acute cardiomyopathy in rats. Hum. Exp. Toxicol. 2011, 30, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N.; Osman, W.M. Aliskiren attenuates bleomycin-induced pulmonary fibrosis in rats: Focus on oxidative stress, advanced glycation end products, and matrix metalloproteinase-9. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 897–909. [Google Scholar] [CrossRef]
- Nakano, Y.; Matoba, T.; Tokutome, M.; Funamoto, D.; Katsuki, S.; Ikeda, G.; Nagaoka, K.; Ishikita, A.; Nakano, K.; Koga, J.I.; et al. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci. Rep. 2016, 6, 29601. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Kwon, Y.M.; Omidi, Y.; Speth, R.C. Nanoparticle approaches for the renin-angiotensin system. Heliyon 2023, 9, e16951. [Google Scholar] [CrossRef]
- Antal, I.; Kubovcikova, M.; Zavisova, V.; Koneracka, M.; Pechanova, O.; Barta, A. Magnetic poly(D,L-lactide) nanoparticles loaded with aliskiren: A promising tool for hypertension treatment. J. Magn. Magn. Mater. 2015, 380, 280–284. [Google Scholar] [CrossRef]
- Behuliak, M.; Vavrínová, A.; Bencze, M.; Polgárová, K.; Ergang, P.; Kuneš, J.; Vanecková, I.; Zicha, J. Ontogenetic changes in contribution of calcium sensitization and calcium entry to blood pressure maintenance of Wistar-Kyoto and spontaneously hypertensive rats. J. Hypertens. 2015, 33, 2443–2454. [Google Scholar] [CrossRef]
- Vavřínová, A.; Behuliak, M.; Bencze, M.; Vodička, M.; Ergang, P.; Vaněčková, I.; Zicha, J. Sympathectomy-induced blood pressure reduction in adult normotensive and hypertensive rats is counteracted by enhanced cardiovascular sensitivity to vasoconstrictors. Hypertens. Res. 2019, 42, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Vavřínová, A.; Behuliak, M.; Zicha, J. The importance of the selection of appropriate reference genes for gene expression profiling in adrenal medulla or sympathetic ganglia of spontaneously hypertensive rat. Physiol. Res. 2016, 65, 401–411. [Google Scholar] [CrossRef]
- Şaman, E.; Cebova, M.; Barta, A.; Koneracka, M.; Zavisova, V.; Eckstein-Andicsova, A.; Danko, M.; Mosnacek, J.; Pechanova, O. Combined therapy with simvastatin- and coenzyme-Q10-loaded nanoparticles upregulates the Akt-eNOS Pathway in Experimental metabolic syndrome. Interantiona J. Mol. Sci. 2022, 24, 276. [Google Scholar] [CrossRef]
- Cebova, M.; Klimentova, J.; Janega, P.; Pechanova, O. Effect of bioactive compound of aronia melanocarpa on cardiovascular system in experimental hypertension. Oxidative Med. Cell. Longev. 2017, 2017, 8156594. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barta, A.; Cebova, M.; Kovac, A.; Koneracka, M.; Zavisova, V.; Pechanova, O. Aliskiren-Loaded Nanoparticles Downregulate (Pro)renin Receptor and ACE Gene Expression in the Heart of Spontaneously Hypertensive Rats: Effect on NADPH Oxidase. Int. J. Mol. Sci. 2024, 25, 846. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25020846
Barta A, Cebova M, Kovac A, Koneracka M, Zavisova V, Pechanova O. Aliskiren-Loaded Nanoparticles Downregulate (Pro)renin Receptor and ACE Gene Expression in the Heart of Spontaneously Hypertensive Rats: Effect on NADPH Oxidase. International Journal of Molecular Sciences. 2024; 25(2):846. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25020846
Chicago/Turabian StyleBarta, Andrej, Martina Cebova, Andrej Kovac, Martina Koneracka, Vlasta Zavisova, and Olga Pechanova. 2024. "Aliskiren-Loaded Nanoparticles Downregulate (Pro)renin Receptor and ACE Gene Expression in the Heart of Spontaneously Hypertensive Rats: Effect on NADPH Oxidase" International Journal of Molecular Sciences 25, no. 2: 846. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25020846