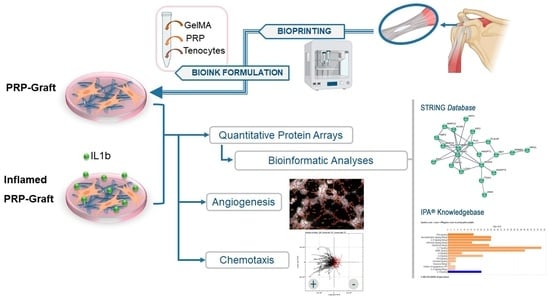
Assessing Bioprinted Functionalized Grafts for Biological Tendon Augmentation In Vitro
Abstract
:1. Introduction
2. Results
2.1. Tendon Stromal Cells Adhesion and Viability within PRP Grafts
Viability of Tendon Cells within Bioprinted Grafts
2.2. Predicted Protein–Protein Interactions and Networks for PRP-Infused Grafts: STRING Database
2.3. Analyses of PRP Grafts in the IPA® Knowledgebase
2.3.1. Activated Canonical Pathways in the IPA® Knowledgebase
2.3.2. Response of PRP Grafts in Inflamed Environments
2.4. Impact of IL-1b Exposure on PRP Grafts: Meaningful Activated Pathways in the Context of Tendinopathy
Matrigel Assay
3. Discussion
4. Methods and Materials
4.1. Primary Culture of Human Tenocytes
4.2. Preparation of PRP
4.3. Preparation of Functionalized GelMA
4.4. Bioprinting of Tendon Grafts
4.5. Tendon Cell Viability within Bioprinted Grafts
4.6. In Vitro Evaluation of Graft Biology with and without Inflammation
4.7. Antibody-Based Protein Arrays
4.8. Bioinformatic Analyses
4.8.1. Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) Database
4.8.2. Ingenuity Pathway Analysis (IPA®)
4.9. Angiogenic Assays
4.9.1. Chemotaxis of HUVEC towards PRP Stimuli
4.9.2. Matrigel Assays: HUVEC Exposed to PRP or Conditioned Media by Inflamed Tenocytes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | conditioned media |
| ECM | extracellular matrix |
| FN | Fibronectin |
| GelMA | methacrylate gelatin |
| HGF | Hepatocyte Growth Factor |
| HIF-1a | Hypoxia Inducible Factor alpha |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| ICAM | Intercellular Adhesion Molecule |
| IFNG | Interferon gamma |
| IGF-1 | insulin-like growth factor |
| IL | Interleukin |
| IPA | Ingenuity Pathway Analyses |
| KDR | Kinase Domain Receptor |
| LAP | Lithium phenyl-2,4,6- Trimethylbenzoylphosphinate |
| MA | methacrylic anhydride |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | Monocyte Chemotactic Protein 1 |
| MMP | Matrix Metalloproteinase |
| NF-kB | nuclear factor kappa light chain enhancer of activated B cells |
| PDGF | Platelet-Derived Growth Factor |
| PF4 | Platelet Factor-4 |
| PPI | protein–protein interactions |
| PPP | platelet-poor plasma |
| PRP | platelet-rich plasma |
| PTGS | Prostaglandin Endoperoxide Synthase |
| RANTES | Regulated Upon Activation, Normal T Cell Expressed And Presumably Secreted |
| SELL | Selectin |
| TGF-β | Transforming Growth Factor |
| TLR | Toll-like Receptors |
| TNF | Tumor Necrosis Factor |
| TRAF | TNF Receptor-Associated Factor |
| TSP-1 | Thrombospondin |
| VCAM | Vascular Cell Adhesion Molecule |
| VEGF | Vascular Endothelial Growth Factor |
References
- Sprague, A.L.; Smith, A.H.; Knox, P.; Pohlig, R.T.; Grävare Silbernagel, K. Modifiable risk factors for patellar tendinopathy in athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.B.; Pizzari, T.; Kinsella, R.; Hope, D.; Cook, J.L. Current trends in tendinopathy management. Best Pract. Res. Clin. Rheumatol. 2019, 33, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.F.; Libertiaux, V.; Dupont, L.; Colige, A.; Denoël, V.; Lecut, C.; Hego, A.; Gustin, M.; Duwez, L.; Oury, C.; et al. Platelet-rich plasma (PRP) and tendon healing: Comparison between fresh and frozen-thawed PRP. Platelets 2020, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Masiello, F.; Pati, I.; Veropalumbo, E.; Pupella, S.; Cruciani, M.; De Angelis, V. Ultrasound-guided injection of platelet-rich plasma for tendinopathies: A systematic review and meta-analysis. Blood Transfus. 2023, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Imasu, H.; Takano, K.; Umezu, M.; Okazaki, K.; Iwasaki, K. Time-series biological responses toward decellularized bovine tendon graft and autograft for 52 consecutive weeks after rat anterior cruciate ligament reconstruction. Sci. Rep. 2022, 12, 6751. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Rubio-Azpeitia, E.; Maffulli, N. Platelet-rich Plasma Modulates the Secretion of Inflammatory/Angiogenic Proteins by Inflamed Tenocytes. Clin. Orthop. Relat. Res. 2015, 473, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Jomaa, G.; Kwan, C.K.; Fu, S.C.; Ling, S.K.K.; Chan, K.M.; Yung, P.S.H.; Rolf, C. A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet. Disord. 2020, 21, 78. [Google Scholar] [CrossRef]
- Stolk, M.; Klatte-Schulz, F.; Schmock, A.; Minkwitz, S.; Wildemann, B.; Seifert, M. New insights into tenocyte-immune cell interplay in an in vitro model of inflammation. Sci. Rep. 2017, 7, 9801. [Google Scholar] [CrossRef]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.; Smith, R.D.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease Europe PMC Funders Group. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef]
- Gholizadeh-Ghaleh Aziz, S.; Alipour, S.; Ranjbarvan, P.; Azari, A.; Babaei, G.; Golchin, A. Critical roles of TLRs on the polarization of mesenchymal stem cells for cell therapy of viral infections: A notice for COVID-19 treatment. Comp. Clin. Path. 2021, 30, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, T.; Lyu, K.; Chen, Y.; Lu, J.; Wang, X.; Long, L.; Li, S. Inflammation-related signaling pathways in tendinopathy. Open Life Sci. 2023, 18, 20220729. [Google Scholar] [CrossRef] [PubMed]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon healing is adversely affected by low-grade inflammation. J. Orthop. Surg. Res. 2021, 16, 700. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, R.; Vuong, I.; Li, F.; Kong, J.; Mao, H.Q. Biomaterials strategies to balance inflammation and tenogenesis for tendon repair. Acta Biomater. 2021, 130, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Bai, L.; Qian, Y.; Zhang, X.; Wang, J.; Zhou, J.; Cui, W.; Hao, Y.; Yang, X. Antioxidant and anti-inflammatory injectable hydrogel microspheres for in situ treatment of tendinopathy. Regen. Biomater. 2024, 11, rbae007. [Google Scholar] [CrossRef] [PubMed]
- Donderwinkel, I.; Tuan, R.S.; Cameron, N.R.; Frith, J.E. A systematic investigation of the effects of TGF-β3 and mechanical stimulation on tenogenic differentiation of mesenchymal stromal cells in a poly(ethylene glycol)/gelatin-based hydrogel. J. Orthop. Transl. 2023, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Luo, H.; Pan, J.; Cui, W.; Cheng, B.; Zhao, S.; Chen, G. Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow stimulation for enthesis healing in rotator cuff repair. J. Shoulder Elb. Surg. 2021, 30, 544–553. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, T.; Zheng, N.; Zhou, Y.; Hogan, M.V.; Wang, J.H.C. The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Jt. Res. 2017, 6, 231–244. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Z.; Bauer, R.J.; Peng, J.; Schieker, M.; Nerlich, M.; Docheva, D. Functionalized thermosensitive hydrogel combined with tendon stem/progenitor cells as injectable cell delivery carrier for tendon tissue engineering. Biomed. Mater. 2018, 13, 034107. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.I.; Atilano, L.; Merino, J.; Gonzalez, I.; Iglesias, G.; Areizaga, L.; Bully, P.; Grandes, G.; Andia, I. Platelet-rich plasma versus lidocaine as tenotomy adjuvants in people with elbow epicondylopathy: A randomized controlled trial. J. Orthop. Surg. Res. 2019, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.I.; Atilano, L.; Bully, P.; Iglesias, G.; Merino, J.; Grandes, G.; Andia, I. Needle tenotomy with PRP versus lidocaine in epicondylopathy: Clinical and ultrasonographic outcomes over twenty months. Skeletal Radiol. 2019, 48, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Bulsara, M.K.; O’Donnell, J.; McCrory, P.R.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: A Randomized, Double-Blind Controlled Trial Comparing a Single Platelet-Rich Plasma Injection With a Single Corticosteroid Injection. Am. J. Sports Med. 2018, 46, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Gilchrist, D.S.; Akbar, M.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Murrell, G.A.C.; Liew, F.Y.; Kurowska-Stolarska, M.; McInnes, I.B. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat. Commun. 2015, 6, 6774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiang, L.; Lin, F.; Tang, Y.; Deng, L.; Cui, W. A Biomaterial-Based Hedging Immune Strategy for Scarless Tendon Healing. Adv. Mater. 2022, 34, 2200789. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, J.Y.; Snelling, S.J.B.; Carr, A.J.; Dakin, S.G. Interleukin-17 Cytokines and Receptors: Potential Amplifiers of Tendon Inflammation. Front. Bioeng. Biotechnol. 2021, 9, 795830. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.J.; Carr, A.J.; Snelling, S.J.B.; Wheway, K.; Watkins, B.; Dakin, S.G. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1α in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc. Med. 2017, 3, e000225. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Melchor, E.; Cafaro, G.; MacDonald, L.; Crowe, L.A.N.; Sood, S.; McLean, M.; Fazzi, U.G.; McInnes, I.B.; Akbar, M.; Millar, N.L. Novel self-amplificatory loop between T cells and tenocytes as a driver of chronicity in tendon disease. Ann. Rheum. Dis. 2021, 80, 1075–1085. [Google Scholar] [CrossRef]
- Akbar, M.; Crowe, L.A.N.; McLean, M.; Garcia-Melchor, E.; MacDonald, L.; Carter, K.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; et al. Translational targeting of inflammation and fibrosis in frozen shoulder: Molecular dissection of the T cell/ IL-17A axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102715118. [Google Scholar] [CrossRef]
- Tuzlak, S.; Dejean, A.S.; Iannacone, M.; Quintana, F.J.; Waisman, A.; Ginhoux, F.; Korn, T.; Becher, B. Repositioning TH cell polarization from single cytokines to complex help. Nat. Immunol. 2021, 22, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, D.; Williamson, K.; Rocha, J.L.; Hogan, M.C.V.; Wang, J.H.C. Selectively activated PRP exerts differential effects on tendon stem/progenitor cells and tendon healing. J. Tissue Eng. 2019, 10, 2041731418820034. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.S.; Smith, R.D.J.; Nagra, N.; Wheway, K.; Watkins, B.; Snelling, S.; Dakin, S.G.; Carr, A.J. Rotator cuff repair with biological graft augmentation causes adverse tissue outcomes. Acta Orthop. 2020, 91, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Yang, W.; Yu, K.; Hong, J.; Ji, X.; Yao, M.; Li, S.; Lu, J.; Chen, Y.; et al. 3D-printed hydrogel particles containing PRP laden with TDSCs promote tendon repair in a rat model of tendinopathy. J. Nanobiotechnol. 2023, 21, 1–14. [Google Scholar] [CrossRef]
- Tsai, W.C.; Yu, T.Y.; Chang, G.J.; Lin, L.P.; Lin, M.S.; Pang, J.H.S. Platelet-Rich Plasma Releasate Promotes Regeneration and Decreases Inflammation and Apoptosis of Injured Skeletal Muscle. Am. J. Sports Med. 2018, 46, 1980–1986. [Google Scholar] [CrossRef]






| PRP Graft | Inflamed PRP Graft | |
|---|---|---|
| HIF1α Signaling | 4.082 | 3.266 |
| Neuroinflammation Signaling Pathway | 3.656 | 2.694 |
| IL-33 Signaling Pathway | 3.53 | 3.024 |
| S100 Family Signaling Pathway | 3.395 | 3.479 |
| Osteoarthritis Pathway | 3.272 | 2.502 |
| IL-17 Signaling | 3.244 | 3.647 |
| IL-8 Signaling | 3 | 2.84 |
| IL-6 Signaling | 2.524 | 2.982 |
| TGF-β Signaling | 2.449 | 1.633 |
| Chemokine Signaling | 2.333 | 3 |
| Inhibition of Angiogenesis by TSP1 | 2 | 2.236 |
| p38 MAPK Signaling | 1.807 | 2.84 |
| NF-kB Pathway | 1.976 | 3.413 |
| VEGF Family Ligand–Receptor Interactions | 0.447 | 2.236 |
| VEGF Signaling | 0.816 | 2.449 |
| CD40 Signaling | 1 | 2 |
| Toll-like Receptor Signaling | 1.265 | 2.111 |
| IL-1 Family Signaling | 0.302 | 2.111 |
| IL-10 Signaling | −2.4 | −2.357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Amo, C.; Perez-Garrastachu, M.; Jauregui, I.; Llama-Pino, X.; Andia, I. Assessing Bioprinted Functionalized Grafts for Biological Tendon Augmentation In Vitro. Int. J. Mol. Sci. 2024, 25, 4752. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25094752
Del Amo C, Perez-Garrastachu M, Jauregui I, Llama-Pino X, Andia I. Assessing Bioprinted Functionalized Grafts for Biological Tendon Augmentation In Vitro. International Journal of Molecular Sciences. 2024; 25(9):4752. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25094752
Chicago/Turabian StyleDel Amo, Cristina, Miguel Perez-Garrastachu, Ines Jauregui, Xabier Llama-Pino, and Isabel Andia. 2024. "Assessing Bioprinted Functionalized Grafts for Biological Tendon Augmentation In Vitro" International Journal of Molecular Sciences 25, no. 9: 4752. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25094752