15N Chemical Shifts in Energetic Materials: CP/MAS and ab Initio Studies of Aminonitropyridines, Aminonitropyrimidines, and Their N-Oxides
Abstract
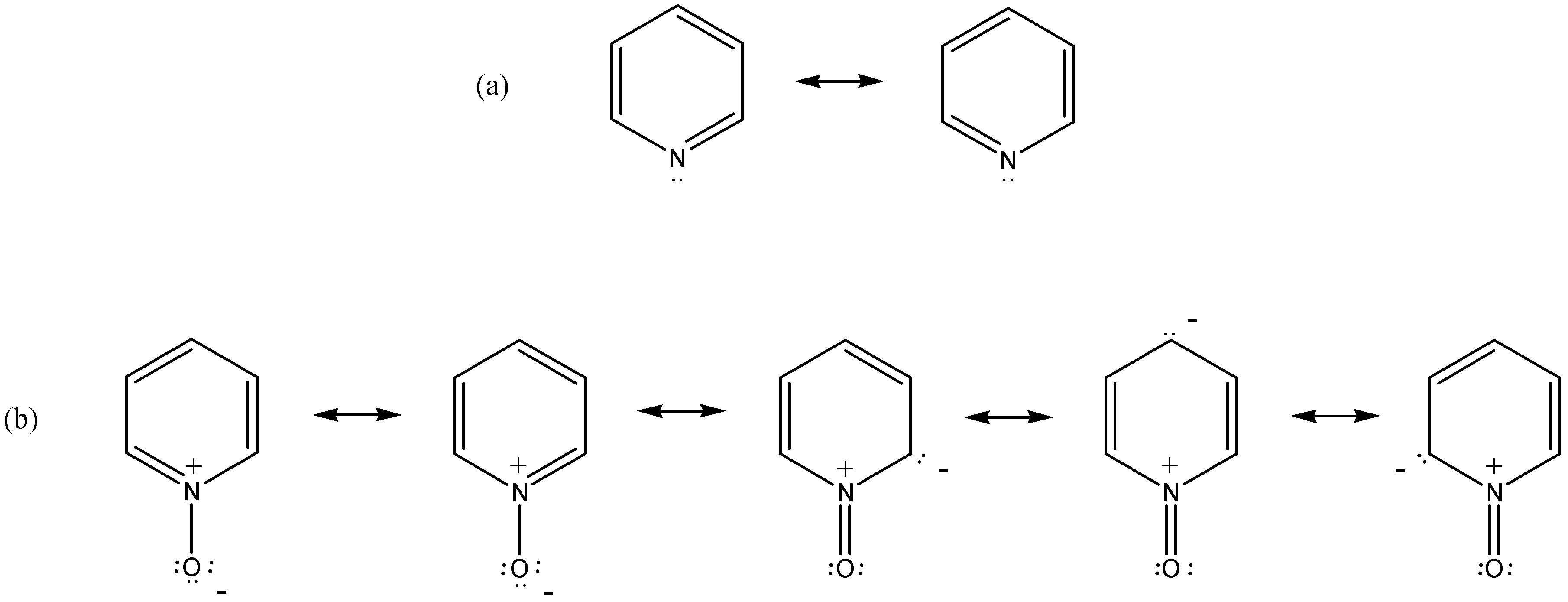
:1. Introduction

2. Experimental and Computational Methods
a) Materials
b) NMR measurements
c) Calculations
- i)
- Optimized Geometries (OPT): The geometries of all the compounds were optimized using the same method described above to calculate the chemical shifts, i.e., DFT using the BLYP exchange correlation functional and the D95** basis set.
- ii)
- iii)
- X-ray Geometries with Hydrogen Bonding (HB): Calculations were performed using the X‑ray geometries with the effects of the nearest neighboring molecules included to account for intermolecular effects, mainly hydrogen bonding, in the shielding calculations. Calculations using this model were completed for the compounds with known X-ray structures (I, II, IV, VI, and VII). To reduce the size of the calculations, only the interacting pieces of the first neighboring molecules were included in the calculations. .pdb files with the geometries used in the calculations are available from the authors (JCF) upon request.
- iv)
- X-ray Geometries with Hydrogen Bonding and Optimized Proton Positions (HB/Hopt): To further improve the model and optimize the contribution due to intermolecular effects, calculations were performed using the geometries in iii, but additionally optimizing the positions of the protons in the model. This procedure was used in view of the well known fact that accurate positions of the protons are necessary to obtain good agreement between the calculated and experimental values of the chemical shifts [32]. The positions of the protons were optimized using the Unified Force Field (UFF) [33,34] as implemented in the Gaussian 98 program.
3. Results and Discussion
| Structure (Nucleus)b | Calculated Values | Exp.c | |||
|---|---|---|---|---|---|
| OPT | XR | HB | HB/Hopt | ||
| I-NO2 (3) | -21 | -43 | -45 | -47 | -13 |
| I-NO2 (5) | -21 | -40 | -45 | -45 | -13 |
| I-N (1) | -165 | -173 | -179 | -180 | -194 |
| I-NH2 (2) | -277 | -317 | -306 | -288 | -277 or -274d |
| I-NH2 (4) | -275 | -313 | -302 | -304 | -267 |
| I-NH2 (6) | -277 | -316 | -307 | -300 | -277 or -274d |
| II-NO2 (3) | -25 | -44 | -47 | -47 | -15 |
| II-NO2 (5) | -25 | -44 | -47 | -48 | -15 |
| II-N-O (1) | -160 | -166 | -170 | -173 | -186 |
| II-NH2 (2) | -285 | -322 | -314 | -295 | -283 or -279d |
| II-NH2 (4) | -277 | -318 | -309 | -296 | -273 |
| II-NH2 (6) | -285 | -322 | -312 | -313 | -283 or -279d |
| III-NO2 (3, 5) | -20 | - | - | - | -13 |
| III-N (1) | -144 | - | - | - | -162 |
| III-NH2 (2, 6) | -280 | - | - | - | -281 |
| IV-NO2 (3) | -21 | -40 | -40 | -42 | -14 |
| IV-NO2 (5) | -21 | -40 | -40 | -44 | -14 |
| IV-N-O (1) | -139 | -153 | -157 | -155 | -159 |
| IV-NH2 (2) | -290 | -328 | -317 | -290 | -292 |
| IV-NH2 (6) | -290 | -328 | -316 | -300 | -292 |
| V-NO2 (5) | -21 | - | - | - | -17 |
| V-N (1, 3) | -176 | - | - | - | -200, -198e |
| V-NH2 (4, 6) | -285 | - | - | - | -280 |
| V-NH2 (2) | -295 | - | - | - | -290 |
| VI-NO2 (5) | -26 | -51 | -53 | -53 | -19 |
| VI-N-O (1) | -159 | -164 | -174 | -176 | -192f |
| VI-N (3) | -193 | -196 | -202 | -205 | -213f |
| VI-NH2 (2) | -300 | -340 | -310 | -298 | -271 |
| VI-NH2 (4) | -294 | -334 | -322 | -310 | -289 |
| VI-NH2 (6) | -286 | -327 | -322 | -307 | -289 |
| VII-NO2 (5) | -28 | -56 | -58 | -60 | -19 |
| VII-N-O (1) | -166 | -171 | -182 | -185 | -198g |
| VII-N-O (3) | -166 | -177 | -180 | -183 | -192 g |
| VII-NH2 (2) | -318 | -339 | -328 | -311 | -291h |
| VII-NH2 (4) | -339 | -331 | -309 | -298 | -291h |
| VII-NH2 (6) | -331 | -318 | -305 | -286 | -291h |
| Model | RMSa | R2 | Slope | Intercepta |
|---|---|---|---|---|
| OPTb | 18.9 | 0.9743 | 1.01 | 0.61 |
| XR | 26.4 | 0.9548 | 1.04 | -16.3 |
| HB | 20.1 | 0.9705 | 0.99 | -22.2 |
| HB/Hopt | 17.2 | 0.9758 | 0.93 | -26.8 |
| Structure (Nucleus) | δ11a | δ22b | δ33c | δisod |
|---|---|---|---|---|
| I (2, 6) | -202 | -307 | -323 | -277 (-277, -274) |
| I(4) | -177 | -289 | -358 | -275 (-267) |
| II (2, 6) | -211 | -302 | -341 | -285 (-279, -283) |
| II(4) | -178 | -290 | -363 | -277 (-273) |
| III (2, 6) | -207 | -312 | -322 | -280 (-281) |
| IV (2, 6) | -220 | -306 | -343 | -290 (-292) |
| V (4, 6) | -211 | -309 | -335 | -285 (-280) |
| V (2) | -247 | -284 | -354 | -295 (-290) |
| VI (4) | -213 | -311 | -333 | -286 (-289) |
| VI (6) | -223 | -315 | -343 | -294 (-289) |
| VI (2) | -243 | -320 | -338 | -300 (-271) |
| VII (4) | -294 | -353 | -371 | -339 (-291) |
| VII (6) | -261 | -354 | -378 | -331 (-291) |
| VII (2) | -248 | -334 | -371 | -318 (-291) |
| Structure (Nucleus) | δ11a | δ22b | δ33c | δisod |
|---|---|---|---|---|
| I (3, 5) | 73 | 36 | -171 | -21 (-13) |
| II (3, 5) | 74 | 21 | -169 | -25 (-15) |
| III (3, 5) | 95 | 17 | -172 | -20 (-13) |
| IV (3, 5) | 99 | 10 | -170 | -21(-14) |
| V (5) | 73 | 36 | -173 | -21 (-17) |
| VI (5) | 74 | 21 | -174 | -26 (-19) |
| VII (5) | 77 | 11 | -172 | -28 (-19) |
| Structure (Nucleus) | δ11a | δ22b | δ33c | δisod |
|---|---|---|---|---|
| N | ||||
| I (1) | -53 | -124 | -319 | -165 (-194) |
| III (1) | -13 | -103 | -315 | -144 (-162) |
| V (1, 3) | -72 | -122 | -335 | -176 (-200, -198)e |
| VI (3) | -92 | -140 | -346 | -193 (-213) |
| N-O | ||||
| II (1) | -90 | -142 | -246 | -160 (-186) |
| IV (1) | -60 | -114 | -242 | -139 (-159) |
| VI (1) | -97 | -132 | -249 | -159 (-192) |
| VII (1) | -99 | -147 | -251 | -177 (-192, -198) |
| Position, Group | Pyridine | Pyridine-1-Oxide |
|---|---|---|
| o-NH2 | -50 (-53.9) | -34 |
| m-NH2 | 0 (-2.7) | 0 |
| p-NH2 | -30 (-39.7) | -18 |
| o-NO2 | -23 | 3 |
| m-NO2 | -4 | 0 |
| p-NO2 | -24 | -10 |
| Structure (Nucleus) | Exp. | Theor.a | Subst. Effectb |
|---|---|---|---|
| I | -194 | -165 | -201 |
| II | -186 | -160 | -184 |
| III | -162 | -144 | -171 |
| IV | -159 | -139 | -166 |
| V | -200 -198 | -176 | -219 |
| VI (N) | -213 | -193 | -214 |
| VI (N-O) | -192 | -159 | -176 |
| VII | -198 -192 | -166 | -176 |
4. Conclusions
Acknowledgments
References
- Cady, H. H.; Larson, A. C. The Crystal Structure of 1,3,5-triamino-2,4,6-trinitrobenzene. Acta Crystallogr. 1965, 18, 485–496. [Google Scholar] [CrossRef]
- Fried, L. E.; Ruggiero, A. J. Energy Transfer Dynamics and Impact Sensitivity. Matl. Res. Soc. Symp. Proc. 1993, 296, 35–40. [Google Scholar] [CrossRef]
- Sharma, J.; Beard, B. C. Chemistry and Physics of Energetic Materials; Bulusu, S., Ed.; Kluwer: Dordrech, 1990; p. 581. [Google Scholar]
- Hollins, R. A.; Merwin, L. H.; Nissan, R. A.; Wilson, W. S.; Gilardi, R. Aminonitropyridines and their N-Oxides. J. Heterocycl. Chem. 1996, 33, 895–904. [Google Scholar] [CrossRef]
- Altmann, K. L.; Merwin, L. H.; Nissan, R. A.; Wilson, W. S. Solid State 13C and 15N NMR Investigations of Novel Pyridines, Pyrimidines, and their N-Oxides. In Poster #263, 37th Rocky Mountain Conference on Analytical Chemistry, Denver, CO, July 23 - 27, 1995.
- Altmann, K. L.; Merwin, L. H.; Nissan, R. A.; Wilson, W. W. Solid State NMR Investigations of Novel Pyridines, Pyrimidines, and their N-Oxides: Reversed Ring-Nitrogen Shift Assignments. In Poster #WP264, 37th Experimental NMR Conference; Asilomar, CA: March 17 ‑ 26, 1996.
- Facelli, J. C.; Grant, D. M. Determination of Molecular Symmetry in Crystalline Naphthalene Using Solid-State NMR Data. Nature 1993, 365, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Witanowski, M.; Stefaniak, L.; Webb, G. A. Nitrogen NMR Spectroscopy. In Annu. Rep. NMR Spectrosc.; Webb, G. A., Ed.; Academic Press: London, 1993; Vol. 25, pp. 1–480. [Google Scholar]
- Mason, J. Nitrogen NMR. In Encyclopedia of Nuclear Magnetic Resonance; Grant, D. M., Harris, R. K., Eds.; John Wiley: London, 1996; p. 3222. [Google Scholar]
- Hu, J. Z.; Zhou, J.; Yang, B.; Li, L.; Qiu, J.; Ye, C.; Pugmire, R. J.; Solum, M. S.; Wind, R.; Grant, D. M. Dynamic Nuclear Polarization of Nitrogen-15. Solid State NMR 1997, 8, 129–137. [Google Scholar] [CrossRef]
- Anderson-Altmann, K. L.; Phung, C. G.; Mavromoustakos, S.; Zheng, Z.; Facelli, J. C.; Poulter, C. D.; Grant, D. M. The 15N Chemical Shift Tensors of Uracil Determined from 15N Powder Pattern and 15N-13C Dipolar NMR Spectroscopy. J. Phys. Chem. 1995, 99, 10454–10458. [Google Scholar] [CrossRef]
- Solum, M. S.; Altmann, K. L.; Strohmeier, M.; Berges, D. A.; Zhang, Y.; Facelli, J. C.; Pugmire, R. J.; Grant, D. M. 15N Chemical Shift Principal Values in Nitrogen Heterocycles. J. Am. Chem. Soc. 1997, 119, 9804–9809. [Google Scholar] [CrossRef]
- Facelli, J. C.; Pugmire, R. J.; Grant, D. M. Effects of Hydrogen Bonding in the Calculation of 15N Chemical Shift Tensors: Benzamide. J. Am. Chem. Soc. 1996, 118, 5488–5489. [Google Scholar] [CrossRef]
- Giessner-Prette, C. J. Ab-Initio Quantum Mechanical Calculations of NMR Chemical Shifts in Nucleic Acid Constituents. I. The Watson-Crick Base Pair. J. Biomol. Struct. Dynamics 1984, 2, 233–248. [Google Scholar] [CrossRef]
- Schindler, M. Magnetic Properties in Terms of Localized Quantities. 9. The DNA Bases, and the Protonation of Adenine. J. Am. Chem. Soc. 1988, 110, 6623–6630. [Google Scholar] [CrossRef]
- Facelli, J. C. Shielding Tensor Calculations. In Encyclopedia of Nuclear Magnetic Resonance; Grant, D. M., Harris, R. K., Eds.; John Wiley: London, 1996; pp. 4327–4334. [Google Scholar]
- Facelli, J. C. Modeling NMR Chemical Shifts: Gaining Insights into Structure and Environment; Facelli, J. C., de Dios, A. C., Eds.; American Chemical Society Symposium Series, Oxford University Press: London, 1999; No. 732. [Google Scholar]
- de Dios, A. C.; Oldfield, E. Recent Progress in Understanding Chemical Shifts. Solid State NMR 1996, 6, 101–125. [Google Scholar] [CrossRef]
- Ferraro, M. B.; Repetto, V.; Facelli, J. C. Modeling NMR Chemical Shifts: A Comparison of Charge Models for Solid State Effects on 15N Chemical Shift Tensors. Solid State NMR 1998, 10, 185–189. [Google Scholar] [CrossRef]
- Delia, T. J.; Portlock, D. E.; Venton, D. L. Pyrimidine N-Oxides. Oxidation of 5-nitroso-2,4,6-triaminopyrimidine. J. Heterocycl. Chem. 1968, 5, 449–451. [Google Scholar] [CrossRef]
- London, F. Quantum Theory of Interatomic Currents in Aromatic Compounds. J. Phys. Radium 1937, 8, 397–409. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-Consistent Perturbation Theory of Diamagnetism. I. A Gage-Invariant LCAO (Linear Combination of Atomic Orbitals) Method for NMR Chemical Shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J. F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Dunning, T. H.; Hay, P. J. Modern Theoretical Chemistry; Schaefer, H. F., III, Ed.; Plenum: New York, 1976; p. 1. [Google Scholar]
- Hohenberg, P.; Khon, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Khon, W.; Sham, L. J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Becke, A. D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar]Lee, C.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Cheeseman, J. R.; Trucks, G. W.; Keith, T. A.; Frisch, M. J. A Comparison of Models for Calculating Nuclear Magnetic Resonance Shielding Tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; C. Peng, Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 98, Rev. A.7; Gaussian, Inc.: Pittsburgh, 1998. [Google Scholar]
- Jameson, J. C.; Mason, J. Multinuclear NMR; Mason, J., Ed.; Plenum: New York, 1987; p. 56. [Google Scholar]
- Gilardi, R. Naval Research Laboratory. unpublished results.
- Grant, D. M.; Liu, F.; Iuliucci, R. J.; Phung, C.G.; Facelli, J. C.; Alderman, D. W. Relationship of 13C Chemical Shift Tensors to Diffraction Structures. Acta Crystallogr., Sect. B 1995, 51, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Rappé, A. K.; Casewit, C. J.; Colwell, K. S.; Goddard, W. A., III; Skiff, W. M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Rappé, A. K.; Goddard, W. A., III. Charge Equilibration for Molecular Dynamics Simulations. J. Phys. Chem. 1991, 95, 3358–3363. [Google Scholar]
- Jameson, C. J.; Osten, H. J. Theoretical Aspects of Isotope Effects on Nuclear Shielding. In Annu. Rep. NMR Spectrosc.; Webb, G. A., Ed.; Academic Press: London, 1986; Vol. 17, pp. 1–78. [Google Scholar]
© 2002 by MDPI (http://www.mdpi.org).
Share and Cite
Anderson, K.L.; Merwin, L.H.; Wilson, W.S.; Facelli, J.C. 15N Chemical Shifts in Energetic Materials: CP/MAS and ab Initio Studies of Aminonitropyridines, Aminonitropyrimidines, and Their N-Oxides. Int. J. Mol. Sci. 2002, 3, 858-872. https://0-doi-org.brum.beds.ac.uk/10.3390/i3080858
Anderson KL, Merwin LH, Wilson WS, Facelli JC. 15N Chemical Shifts in Energetic Materials: CP/MAS and ab Initio Studies of Aminonitropyridines, Aminonitropyrimidines, and Their N-Oxides. International Journal of Molecular Sciences. 2002; 3(8):858-872. https://0-doi-org.brum.beds.ac.uk/10.3390/i3080858
Chicago/Turabian StyleAnderson, Karen L., Lawrence H. Merwin, William S. Wilson, and Julio C. Facelli. 2002. "15N Chemical Shifts in Energetic Materials: CP/MAS and ab Initio Studies of Aminonitropyridines, Aminonitropyrimidines, and Their N-Oxides" International Journal of Molecular Sciences 3, no. 8: 858-872. https://0-doi-org.brum.beds.ac.uk/10.3390/i3080858




