1. Introduction
Little is known about the influence of shifts in landscape configuration on the levels of gene flow and genetic drift in plant populations [
1]. The effects of habitat fragmentation are often confused with those of the matrix type and composition across a landscape [
2], but previous studies suggest that open areas in the landscape may affect gene flow [
3,
4] and, in combination with limited dispersion capacity, can intensify distance isolation, accentuating inbreeding rates [
5]. On the other hand, there is a complex relationship between habitat loss and genetic drift. The additional loss of habitat results in a threshold, after which the time needed for the allele fixation decreases rapidly [
6].
Understanding the effects of landscape modifications is crucial, given that habitat loss and fragmentation are the principal drivers of the ongoing loss of biodiversity [
7]. The genetic diversity of a population may decline through the loss of allelic richness, rare alleles, and heterozygosity, reaching an extreme through the fixation of alleles, indicating increased levels of inbreeding in plant populations [
8,
9,
10], because when population fragmentation reduces effective size many ecological processes may be disrupted, such as pollination and seed dispersal, reducing contemporary gene flow and augmenting the risk of extinction due to multiple Allee effects (see [
11]), characterized by correlating population size—or density—with fitness.
So, in light of this evidence, we evaluated the effects of the landscape matrix on the genetic diversity and the levels of inbreeding in the plants of the Cerrado savannah biome. This biome was chosen because, although it is the largest savannah in the world, with an enormous complexity of habitats [
12], its landscape has suffered profound transformations over the past few decades. Originally covering much of Brazil’s central plateau [
13], this savannah has gradually been replaced by farmland, pasture, and urban zones over the past 50 years [
14]. Between 2003 and 2013, in fact, the proportion of the biome covered by agricultural land almost doubled, with 74% of this area being established on intact Cerrado vegetation [
15]. By the end of the 20th century, the extensive impacts that threaten the survival of endemic species and the maintenance of ecosystem services, such as stabilizing the water regime and providing refuge for many species (see [
16]), led to the inclusion of the biome in the world’s biodiversity hotspots [
17]. The fragmentation and loss of habitat increase spatial isolation, reducing the size of populations and interrupting their connectivity through limitations to dispersal [
18], which ultimately leads to a reduction in gene flow and a subsequent decline in genetic diversity [
19]. Previous studies have shown this relationship between fragmentation and increased levels of inbreeding in plant populations in the Cerrado, e.g., [
20,
21]. In addition to landscape processes, research has shown that plants may also be affected directly by climate change.
Climate influences genetic diversity by inducing changes in the distribution of species and acting as a selective factor, with adverse conditions acting as effective barriers to colonization and gene flow, e.g., [
22,
23]. Collevatti et al. [
24] showed that climatic change may affect the distribution and genetic diversity of
Caryocar brasiliensis in the Cerrado biome. However, phylogeographic patterns tend to be species-specific, rather than universal [
25,
26,
27,
28,
29], which led us to decide to evaluate the effects of current climate conditions on the genetic diversity and inbreeding coefficients of the Cerrado populations of different plant species. Despite the taxon specificity of these patterns, we also evaluate the hypothesis that the patterns of distribution of the genetic diversity of these plants are consistent with the center–periphery model (see [
30,
31,
32,
33,
34]).
Given this, we propose the hypothesis that the Cerrado plant species have higher genetic diversity and lower inbreeding towards the geographical center of the biome, based on the convention that population size decreases and spatial isolation increases from the center to the periphery of a species’ range, often because of the decreasing quality of habitats toward the edge of the range [
19,
35]. In this scenario, demographic bottlenecks, varying selection pressures, and restricted gene flow will result in genetic impoverishment, pronounced genetic differentiation among peripheral populations, and substantial divergence from more central populations [
34,
36,
37,
38].
Reliable data on the genetic diversity and population structure of species, and the factors that determine their variation within the species’ ranges have proven to be crucial for planning conservation strategies [
39]. The value of conservation and the allocation of resources for the preservation of marginal species are still subjects of ample debate [
40]. However, it is clear that strong selective pressures caused by stressful conditions can support the acquisitions of new adaptations in marginal areas, conferring a distinct evolutionary potential on these populations [
41]. Consequently, our main objective was to evaluate whether the distribution patterns of the genetic diversity of plants in the Cerrado are consistent with the center–periphery model, contributing to understand the population dynamics of these species. Alternatively, concerned with the high fragmentation rate of this biome, we seek to understand whether the genetic variation patterns of native plants can be affected by landscape and climate characteristics, within a conservationist perspective.
3. Results
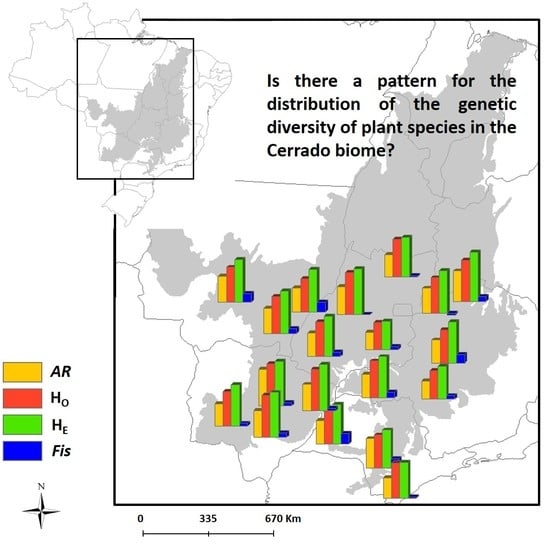
In the present study, literature data were extracted for 31 plant species, totaling 122 populations distributed throughout the Cerrado (
Table 3 and
Table S1), with overall means of H
O = 0.578, H
E = 0.684, and
AR = 10.018. Low mean genetic diversity was observed in
Oryza glumaepatula (H
O = 0.078; H
E = 0.211;
AR = 1.572),
Manihot esculenta (H
O = 0.315; H
E = 0.568;
AR = 3.551),
Dipteryx alata (H
O = 0.333; H
E = 0.418;
AR = 3.312), and
Metrodorea nigra (H
O = 0.353; H
E = 0.588;
AR = 4.000). High inbreeding coefficients were recorded in these species, with the highest values being found in
O. glumaepatula (
Fis = 0.667),
M. esculenta (
Fis = 0.435), and
M. nigra (
Fis = 0.403).
By contrast, the highest genetic diversity values were recorded in Handroanthus chrysotrichus (HO = 0.888; HE = 0.906; AR = 15.000), Annona crassiflora (HO = 0.766; HE = 0.842; AR = 17.650), Tabebuia aurea (HO = 0.765; HE = 0.947; AR = 36.000), and Caryocar brasiliense (HO = 0.764; HO = 0.874; AR = 16.100). Low fixation rates were observed in Plathymenia reticulata (Fis = 0.013), H. chrysotrichus (Fis = 0.021), and Annona coriacea (Fis = 0.022). Negative values were recorded for the fixation index in some species, that is, Solanum lycocarpum (Fis = −0.133), Eugenia dysenterica (Fis = −0.062), Campomanesia adamantium (Fis = −0.030), and Qualea parviflora (Fis = −0.015).
3.1. Spatial Patterns of Genetic Diversity and Structure
We found a clear center–periphery pattern in the spatial distribution of the genetic parameters in
Anonna coriacea and
A. crassiflora (
Figure 1). The triangular pattern observed for the quantiles reveals that the genetic diversity (H
O, H
E, and
AR) of these species is higher in the center of the biome. As expected, additionally, inbreeding rates (
Fis) are higher at the edge of the biome, with reduced genetic diversity in these areas. To visualize the trends and the significance of each regression by species, see
Table S2.
In
Campomanesia adamantium, we observed higher genetic diversity values (H
O, H
E, and
AR) in the populations located closer to the edge of the biome. Given this, we found lower levels of inbreeding in the edge regions (
Figure S1).
In
C. langsdorffii, as in
Annona, genetic diversity tended to decrease with the increasing distance of the populations from the centroid of the Cerrado and, as expected, higher inbreeding rates were observed in the more outlying populations (
Figure S2).
When we analyzed the data on
Dimorphandra mollis, we found no clear evidence of a center–periphery pattern and there is a divergence in relation to the quantile 0.99 for H
O (
Figure S3). For H
E, the pattern was consistent with an increase in this index, in populations located closer to the Cerrado border. Thus, a pattern similar to H
E was observed for
AR and also for
Fis.
In the case of the data available on
Dipteryx alata, we observed a center–periphery pattern only in H
E, that is, the expected heterozygosity showed a tendency to decrease with increasing distance of the populations from the biome’s centroid, which was demonstrated by the 0.99 quantile. The quantiles indicated different patterns for the H
O and
AR data (
Figure S4), although no systematic variation was found in the
Fis.
The populations of
Eugenia dysenterica presented a clear center-periphery pattern in their genetic diversity, with the H
O, H
E, and
AR values all decreasing with increasing distance of the population from the Cerrado centroid (
Figure S5). However, the inbreeding coefficient does not follow the expected pattern, with one quantile showing an increase in the
Fis at the edge of the Cerrado, whereas the other reflected a decrease in this coefficient toward the edge of the biome
Fis.
A relatively well-defined central–peripheral pattern was also found in the genetic diversity of the
Hancornia populations, with the H
O and
AR values indicating significant structuring of the genetic diversity of these species. This is reflected in the higher inbreeding rates found in the more peripheral populations in the biome (
Figure S6).
We analyzed the genera
Handroanthus and
Tabebuia together, given that their species represent the Tabebuia Alliance clade of Grose and Olmstead [
59]. The H
O and H
E values presented a clear center–periphery pattern, which was reinforced by the higher
Fis values observed in the populations at the edge of the Cerrado biome (
Figure S7).
We also observed a clear center–periphery pattern in the H
E and
AR data obtained for
Manihot esculenta, although the highest
Fis values are highest in the populations located in the center of the biome. While this contradicts the pattern observed in the H
E and
AR, it is consistent with the H
O, which was higher toward the edge of the Cerrado (
Figure S8).
The center–periphery pattern was also clear in
Oryza glumepatula, with the indices of genetic diversity (H
O, H
E and
AR) decreasing with increasing distance from the center of the biome, while the
Fis increased with the increasing distance of the populations, at least to the quantile 0.99 (
Figure S9).
By contrast, in the case of the genus
Qualea, one quantile indicated a tendency for H
O and
AR to decrease with increasing distance from the centroid, although this is consistent with the inbreeding coefficient, which tended to be higher in populations over the edge (
Figure S10).
In the genus
Solanum, we verified a clear center–periphery pattern only in the
AR, which decreased with increasing distance from the center of the Cerrado (
Figure S11).
We observed a clear center–periphery pattern in the H
E and
AR of the
Vellozia gigantea populations, with genetic diversity decreasing with increasing distance from the Cerrado centroid. However, the inbreeding coefficient also followed this pattern, that is, it decreased with increasing distance from the centroid, which is consistent only with the pattern observed in H
O, which increased towards the periphery of the biome (
Figure S12).
When we analyzed the whole dataset, that is, all the plant species together (
Figure 2), we found center–periphery patterns in the H
O, H
E, and
AR values only for the 0.99 quantile. A similar pattern was verified for
Fis (0.05 and 0.99).
3.2. Effects of Landscape and Climate on Genetic Diversity
When we analyzed the landscape effects, the models were only significant when we excluded the model that incorporates only the species and landscape matrix. When this model was excluded, the percentage of forestry (FO) in the landscape had a significant effect on the H
O patterns in all three buffers, with ∆AICc = 0.0 in all cases, and wAIC = 0.7720 at 1 km, wAIC = 0.6330 at 3 km, and wAIC = 0.6410 at 5 km (
Table 4). By contrast, H
E was related to the percentage of water (WA) in the 1 km buffer (∆AICc = 0.0; wAIC = 0.9120). The incorporation of WA in the model was also more likely to explain H
E in the 3 km buffer (∆AICc = 0.0; wAIC = 0.3300), although in this case, other environmental variables were also capable of explaining the patterns of this index, including VR (∆AICc = 1.3; wAIC = 0.1700), FO (∆AICc = 1.5; wAIC = 0.1590), and AG (∆AICc = 1.6; wAIC = 0.1450). Similarly, for the 5 km buffer, WA provided the best explanation for the observed pattern (∆AICc = 0.0; wAIC = 0.3550), although it was matched by FO (∆AICc = 0.6; wAIC = 0.2680) and AG (∆AICc = 1.7; wAIC = 0.1500).
In the analysis of the landscape effects on the allelic frequency (
AR) in the Cerrado plant species, the model that includes the percentage of urban area (UA) best explains the pattern observed in all three buffers, with ∆AICc = 0.0 in all cases, and wAIC = 0.3820 at 1 km, wAIC = 0.3350 at 3 km, and wAIC = 0.3600 at 5 km (
Table 5). However, the percentage of water (WA) also had a significant effect on the
AR, with ∆AICc = 0.5 and wAIC = 0.3000 at 1 km, ∆AICc = 0.9 and wAIC = 0.2050 at 3 km, and ∆AICc = 0.5 and wAIC = 0.2800 at 5 km (
Table 5). In the 1 km buffer, the third most important model was that of pasture (PA), with ∆AICc = 1.4 and wAIC = 0.1930, whereas at 3 km, it was farmland (AG), with ∆AICc = 1.1 and wAIC = 0.1920, and at 5 km, it was forestry (FO), with ∆AICc = 1.3 and wAIC = 0.1920. The inbreeding coefficient (
Fis) was most affected by the percentage of FO in the landscape assessed in the 1 km buffer (∆AICc = 0.0 and wAIC = 0.6100), whereas the percentage of UA was the most important variable at 3 km (∆AICc = 0.0; wAIC = 0.6920) and 5 km (∆AICc = 0.0; wAIC = 0.5190).
In general, FO, WA, and UA affected the genetic diversity of the plant populations evaluated in the present study (
Figure 3). When we related landscape parameters to the genetic diversity data, it was possible to determine that populations with low H
O, H
E and
AR values and high inbreeding coefficients tend to be associated with highly modified landscapes. For example, the populations of
O. glumaepatula, are surrounded by a landscape dominated by pasture, while those of
M. esculenta occur in areas dominated by urbanization, farmland, and pasture, with few vegetation remnants. A similar pattern was observed in
D. alata. In
Annona and
Handroanthus/
Tabebuia, by contrast, high percentages of remnants of natural vegetation were associated with high H
O, H
E, and
AR values.
When we evaluated the effects of the climatic variables associated with the landscape matrix, the mean annual temperature and the isothermality were the principal factors determining the observed genetic patterns. The mean annual temperature MAT has a significant effect on both the H
O (∆AICc = 0.0; wAIC = 0.9949) and the H
E (∆AICc = 0.0; wAIC = 0.7116), whereas the
AR was affected by the combination of all the climate variables analyzed, with ∆AICc = 0.0 and wAIC = 0.7116 (
Table 6). The patterns of inbreeding can be accounted for by ISO (∆AICc = 0.0; wAIC = 0.3530), MAT (∆AICc = 0.8; wAIC = 0.2380), and also by the model that incorporates only the species and landscape matrix (∆AICc = 1.3; wAIC = 0.1870).
When we analyzed the influence of the landscape and climatic characters, together with the geographical distance between populations, on the genetic diversity variables, we found that the percentage of WA was the most important variable, in the 1 km buffer, related to the differences in genetic diversity observed among the populations (
Table 7). In the 3 km buffer, the geographic distance affected the H
O and
Fis, although the H
E and
AR were not influenced by any of the factors analyzed. In the 5 km buffer, geographic distance affected H
O and
Fis, although the H
O was also affected by the percentage of FO, whereas
AR was affected by the UA on the landscape. In the case of the climatic variables, the results were similar to those observed in GLMM, that is, the differences observed in the MAT and isothermality (ISO) in the areas occupied by the populations contribute to the variation observed in the genetic diversity.
4. Discussion
The central–peripheral model of genetic diversity was in fact observed in many of the plant species evaluated in the present study, which tended to have higher heterozygosity and allelic richness in the more centrally-located populations, which also tended to have lower levels of inbreeding. This pattern likely reflects a decline in the adaptability of the plants to the abiotic conditions found toward the external limits of the biome. The marginal areas of a species’ range tend to be a zone of transition, where selection pressures are generally more intense [
31,
60,
61]. In this case, species with less phenotypic plasticity will become more stressed physiologically, e.g., [
62], which would result in smaller populations, with reduced genetic diversity driven by low gene flow, genetic drift, inbreeding, and directional selection, leading to a marked genetic structure, e.g., [
63,
64,
65,
66]. Marginal areas have a number of challenges, including unfavorable abiotic conditions and competition with other species from other biomes [
67], and the reduced fitness of the plant may lead to larger fluctuations in population size, reducing effective population size in comparison with larger or more stable populations [
68,
69,
70,
71].
The distribution of both
Anonna crassiflora and
A. coriacea is associated with typical Cerrado environments, that is, nutrient-poor latosols [
72].
Hancornia speciosa occurs in the cerrado and cerradão physiognomies, and is thus also associated with poor soils [
73], and a similar distribution pattern was observed in
E. dysenterica, which inhabits poor, sandy, and acidic soils, predominantly in the Cerrado region and on coastal plains [
74]. These preferences, combined with the modifications provoked by the changes in climate occurring during the Pleistocene, have influenced the distribution patterns of these species in the Cerrado biome, in particular, the greater concentration of genetic diversity in the centroid of the biome. Correa Ribeiro et al. [
75] concluded that the area to the north of the center of the Cerrado, in the central Goiás highlands, acted as a refuge for the
A. coriacea populations during the Pleistocene. Collevatti et al. [
76] also showed that a large area of central Brazil, which coincides approximately with the central Cerrado, served as a historical refuge for the
H. speciosa populations during long-term climate change.
The patterns observed in the heterozygosity of the Cerrado plant populations were influenced by the matrix in which the populations were located, with the greatest effects being observed in the landscapes dominated by FO, WA, and UA. However, the effect appears to be positive when the natural vegetation is replaced by areas of preservation and restoration of native forests, rather than farmland or pasture. This is consistent with the fact that woody plants, especially fruiting trees, will attract pollinators and dispersers. Pollinators, in particular, will transit between cultivated areas and natural environments [
77], and the natural landscape provides an important refuge for a diversity of pollinators which provide pollination services in forestry plantations [
78]. Dispersing animals may be attracted to fragments of vegetation close to forestry plantations where they can obtain food. There is also considerable evidence that the crop matrix can provide habitats that support many animal species [
79]. These movements of pollinators and dispersers between different habitats within the matrix may thus contribute to the genetic diversity of the plant populations sampled near these areas.
In buffers where the percentage of WA was greater, H
E tended to decrease, which is consistent with barrier isolation models. Aquatic environments can create discrete barriers, such as waterfalls and reservoirs, which reduce gene flow between the populations located at their margins, e.g., [
80]. However, the river’s slope and the physical–chemical dissimilarities of water can also act as barriers to gene flow and cause detectable differentiation in the genetic constitution of different populations [
81,
82].
Urban areas also appear to affect the genetic diversity of the plant species evaluated. Some populations sampled in buffers with a high percentage of the urban development presented higher levels of diversity, such as
C. brasilienses and
T. aurea. Urban areas surrounded by fragments of vegetation can function as barriers to gene flow [
83], although they may also facilitate dispersion among populations, leading to greater genetic diversity overall, and reduced differentiation between populations of species that are attracted to urban areas [
84]. Many native pollinators, such as bats and bees, may benefit from urban forest resources [
85,
86], but they usually return to the fragments of native vegetation associated with these areas, thus increasing the flow of pollen between fragments, or even between the urban zone and the fragments. The
C. brasilienses populations found in urban areas may be pollinated by the glossophagine bats,
Glossophaga soricina and
Anoura geoffroyi [
87], while
T. aurea is pollinated by bees of the genus
Centris [
88]. These populations can serve as a source of alleles for populations located in fragments of natural vegetation in the area surrounding the urban zone.
Geographical distance affected the variation in the H
O and
Fis values recorded in the present study, indicating that distance is an important landscape component in the determination of the genetic diversity and structure of plant populations in the Cerrado. These findings are consistent with the previous studies that have verified the occurrence of isolation by the distance between populations of a number of Cerrado plant species, e.g., [
89,
90,
91,
92].
The mean annual temperature (MAT) was the principal climatic variable influencing the H
O, H
E, and
Fis, although isothermality (ISO) also affected genetic diversity. Bonte et al. [
93] showed that temperature can determine the limits of the geographical ranges of a species, principally by affecting the ability of the plants and juveniles to disperse and become recruited successfully. The gametocytes are especially sensitive to fluctuations in temperature, both during their development, before pollination, and in the post-pollination stage [
94]. To avoid negative impacts on their reproduction and physiology [
95], plants tend to disperse to regions that correspond to their optimal temperature range [
96]. The more central regions of the biomes in which these species occur may encompass this optimal temperature range and other abiotic conditions appropriate for the species, possibly reflecting a relationship between optimal suitability and the typical climatic conditions of the biome. Our model thus supports the hypothesis that current and future climate change will impact the genetic diversity of many plant populations [
97,
98,
99], given that some species, especially those that are already threatened or restricted to habitats created by climate change, are vulnerable to the loss of specific alleles through inbreeding [
100]. Combined with climate change, landscape modifications need to be considered carefully in order to guarantee the survival and conservation of the genetic diversity of plant species in Cerrado ecosystems, since they represent the main mechanism for maintaining the food webs.
This study includes patterns for a few species in the Cerrado biome, or even for a few populations of these species, which may be improved in the future, if genetic data for other populations or other plants are available in the literature. Likewise, updated information on land use and land cover in this biome will allow us in the future to establish levels of change at different points in the landscape and assess whether there is a real loss of contemporary gene flow in the most modified areas over time.