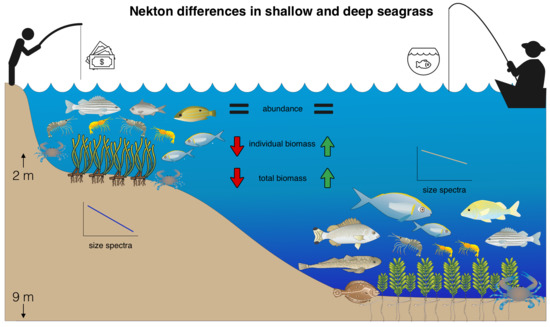
The Differential Importance of Deep and Shallow Seagrass to Nekton Assemblages of the Great Barrier Reef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seagrass Metrics
2.2. Nekton Sampling
2.3. Statistical Analysis
2.4. Size Spectra Analysis
2.5. Determination of Socioeconomic Species
3. Results
3.1. Effects of Seagrass Meadow Depth on Nekton Species Biomass, CPUE and Diversity
3.2. Effects of Seagrass Meadow Depth on Nekton Assemblages
3.3. Size Spectra Analysis
3.4. Socioeconomically Important Fisheries Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coles, R.G.; Lee Long, W.J.; Helmke, S.A.; Bennett, R.E.; Miller, K.J.; Derbyshire, K.J. Seagrass beds and juvenile prawn and fish nursery grounds: Cairns to Bowen. In Queensland Department of Primary Industries Information Series QI92012; Department of Primary Industries: Cairns, Australia, 1992; p. 64. [Google Scholar]
- Coles, R.G.; Lee Long, W.J.; Squire, L.C. Seagrass beds and prawn nursery grounds between Cape York and Cairns. In Queensland Department of Primary Industries Information Series QI85017; Department of Primary Industries: Cairns, Australia, 1985; p. 31. [Google Scholar]
- Coles, R.G.; Mellors, J.; Bibby, J.; Squires, B. Seagrass beds and juvenile nursery grounds between Bowen and Water Park Point. In Queensland Department of Primary Industries Information Series QI87021; Department of Primary Industries: Cairns, Australia, 1987; p. 54. [Google Scholar]
- Coles, R.; McKenzie, L.; De’Ath, G.; Roelofs, A.; Long, W.L. Spatial distribution of deepwater seagrass in the inter-reef lagoon of the Great Barrier Reef World Heritage Area. Mar. Ecol. Prog. Ser. 2009, 392, 57–68. [Google Scholar] [CrossRef]
- Coles, R.G.; Rasheed, M.A.; McKenzie, L.J.; Grech, A.; York, P.H.; Sheaves, M.; McKenna, S.; Bryant, C.; McKenzie, L.J. The Great Barrier Reef World Heritage Area seagrasses: Managing this iconic Australian ecosystem resource for the future. Estuar. Coast. Shelf Sci. 2015, 153, A1–A12. [Google Scholar] [CrossRef]
- York, P.H.; Macreadie, P.I.; Rasheed, M.A. Blue Carbon stocks of Great Barrier Reef deep-water seagrasses. Biol. Lett. 2018, 14, 20180529. [Google Scholar] [CrossRef] [Green Version]
- Long, W.J.L.; Coles, R.G.; McKenzie, L. Issues for seagrass conservation management in Queensland. Pac. Conserv. Biol. 1999, 5, 321–328. [Google Scholar] [CrossRef]
- Rasheed, M.A.; Unsworth, R.K.F. Long-term climate-associated dynamics of a tropical seagrass meadow: Implications for the future. Mar. Ecol. Prog. Ser. 2011, 422, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Grech, A.; Chartrand, K.; Erftemeijer, P.; Fonseca, M.; McKenzie, L.; Rasheed, M.A.; Taylor, H.; Coles, R.; McKenzie, L. A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environ. Res. Lett. 2012, 7, 024006. [Google Scholar] [CrossRef]
- York, P.H.; Carter, A.B.; Chartrand, K.; Sankey, T.; Wells, L.; Rasheed, M.A. Dynamics of a deep-water seagrass population on the Great Barrier Reef: Annual occurrence and response to a major dredging program. Sci. Rep. 2015, 5, 13167. [Google Scholar] [CrossRef]
- Short, F.T.; Neckles, H.A. The effects of global climate change on seagrasses. Aquat. Bot. 1999, 63, 169–196. [Google Scholar] [CrossRef]
- Waycott, M.; Collier, C.; McMahon, K.; Ralph, P.J.; McKenzie, L.J.; Udy, J.W.; Grech, A. Vulnerability of seagrasses in the Great Barrier Reef to climate change. In Climate Change and the Great Barrier Reef: A Vulnerability Assessment; Johnson, J.E., Marshall, P.A., Eds.; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2007; pp. 193–236. [Google Scholar]
- Saunders, M.I.; Leon, J.X.; Callaghan, D.; Roelfsema, C.M.; Hamylton, S.; Brown, C.J.; Baldock, T.E.; Golshani, A.; Phinn, S.R.; Lovelock, C.E.; et al. Interdependency of tropical marine ecosystems in response to climate change. Nat. Clim. Chang. 2014, 4, 724–729. [Google Scholar] [CrossRef]
- Shields, E.C.; Moore, K.A.; Parrish, D.B. Adaptations by zostera marina dominated seagrass meadows in response to water quality and climate forcing. Diversity 2018, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- York, P.H.; Smith, T.; Coles, R.; McKenna, S.A.; Connolly, R.M.; Irving, A.; Jackson, E.L.; McMahon, K.; Runcie, J.W.; Sherman, C.D.H.; et al. Identifying knowledge gaps in seagrass research and management: An Australian perspective. Mar. Environ. Res. 2017, 127, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, R.A.; Coles, R.; Long, W.L. Simulation estimates of annual yield and landed value for commercial penaeid prawns from a tropical seagrass habitat, Northern Queensland, Australia. Mar. Freshwater Res. 1993, 44, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Coles, R.; Long, W.L.; Watson, R.A.; Derbyshire, K. Distribution of seagrasses, and their fish and penaeid prawn communities, in Cairns harbour, a tropical estuary, Northern Queensland, Australia. Mar. Freshwater Res. 1993, 44, 193–210. [Google Scholar] [CrossRef]
- Jinks, K.I.; Brown, C.J.; Rasheed, M.A.; Scott, A.L.; Sheaves, M.; York, P.H.; Connolly, R.M. Habitat complexity influences the structure of food webs in Great Barrier Reef seagrass meadows. Ecosphere 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Francour, P. Fish assemblages of posidonia oceanica beds at Port-Cros (France, NW Mediterranean): Assessment of composition and long-term fluctuations by visual census. Mar. Ecol. 1997, 18, 157–173. [Google Scholar] [CrossRef]
- Heithaus, M.R. Fish communities of subtropical seagrass meadows and associated habitats in Shark Bay, western Australia. Bull. Mar. Sci. 2004, 75, 79–99. [Google Scholar]
- Hutchinson, N.; Jenkins, G.P.; Brown, A.; Smith, T.M. Variation with depth in temperate seagrass-associated fish assemblages in southern Victoria, Australia. Estuaries Coasts 2013, 37, 801–814. [Google Scholar] [CrossRef]
- Pogoreutz, C.; Kneer, D.; Litaay, M.; Asmus, H.; Ahnelt, H. The influence of canopy structure and tidal level on fish assemblages in tropical Southeast Asian seagrass meadows. Estuar. Coast. Shelf Sci. 2012, 107, 58–68. [Google Scholar] [CrossRef]
- Smith, T.; Jenkins, G.P.; Hutchinson, N. Seagrass edge effects on fish assemblages in deep and shallow habitats. Estuar. Coast. Shelf Sci. 2012, 115, 291–299. [Google Scholar] [CrossRef]
- Travers, M.J.; Potter, I.C. Factors influencing the characteristics of fish assemblages in a large subtropical marine embayment. J. Fish. Biol. 2002, 61, 764–784. [Google Scholar] [CrossRef]
- Bell, J.D.; Ferrell, D.J.; McNeill, S.E.; Worthington, D.G. Variation in assemblages of fish associated with deep and shallow margins of the seagrass Posidonia australis. Mar. Biol. 1992, 114, 667–676. [Google Scholar] [CrossRef]
- Jackson, E.; Attrill, M.; Rowden, A.; Jones, M. Seagrass complexity hierarchies: Influence on fish groups around the coast of Jersey (English Channel). J. Exp. Mar. Biol. Ecol. 2006, 330, 38–54. [Google Scholar] [CrossRef]
- Trebilco, R.; Baum, J.K.; Salomon, A.K.; Dulvy, N.K. Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol. Evol. 2013, 28, 423–431. [Google Scholar] [CrossRef]
- Perry, D.; Staveley, T.A.; Gullstrom, M. Habitat connectivity of fish in temperate shallow-water seascapes. Front. Mar. Sci. 2018, 4, 440. [Google Scholar] [CrossRef] [Green Version]
- Sheaves, M. Nature and consequences of biological connectivity in mangrove systems. Mar. Ecol. Prog. Ser. 2005, 302, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Cappo, M.; Kelley, R. Connectivity in the Great Barrier Reef World Heritage Area—An overview of pathways and processes. In Oceanographic Processes of Coral Reefs: Physical and Biological Links in the Great Barrier Reef; Wolanski, E., Ed.; CRC Press: New York, NY, USA, 2000; pp. 161–187. [Google Scholar]
- Nagelkerken, I.; Sheaves, M.; Baker, R.; Connolly, R.M. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish. Fish. 2013, 16, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Sheaves, M. Are there really few piscivorous fishes in shallow estuarine habitats? Mar. Ecol. Prog. Ser. 2001, 222, 279–290. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; De Grave, S.; Jompa, J.; Smith, D.J.; Bell, J.J. Faunal relationships with seagrass habitat structure: A case study using shrimp from the Indo-Pacific. Mar. Freshwater Res. 2007, 58, 1008–1018. [Google Scholar] [CrossRef]
- Unsworth, R.K.; Taylor, J.D.; Powell, A.; Bell, J.J.; Smith, D.J. The contribution of scarid herbivory to seagrass ecosystem dynamics in the Indo-Pacific. Estuar. Coast. Shelf Sci. 2007, 74, 53–62. [Google Scholar] [CrossRef]
- Guest, M.A.; Connolly, R.M.; Loneragan, N.R. Seine nets and beam trawls compared by day and night for sampling fish and crustaceans in shallow seagrass habitat. Fish. Res. 2003, 64, 185–196. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Fishbase 2000: Concepts, Design and Data Sources; ICLARM: Laguna, Philippines, 2000; p. 344. [Google Scholar]
- Clarke, K.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd. Plymouth Marine Laboratory: Plymouth, UK, 2014. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. PERMANOVA+ For PRIMER. Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2008. [Google Scholar]
- Edwards, A.M.; Robinson, J.P.W.; Plank, M.J.; Baum, J.K.; Blanchard, J.L. Testing and recommending methods for fitting size spectra to data. Methods Ecol. Evol. 2016, 8, 57–67. [Google Scholar] [CrossRef]
- White, E.P.; Enquist, B.J.; Green, J.L. On estimating the exponent of power-law frequency distributions. Ecology 2008, 89, 905–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, S.; Mackinson, S. Abundance-body mass relationships in size-structured food webs. Ecol. Lett. 2003, 6, 971–974. [Google Scholar] [CrossRef]
- Jennings, S.; Warr, K.J. Smaller predator-prey body size ratios in longer food chains. R. Soc. B Biol. Sci. 2003, 270, 1413–1417. [Google Scholar] [CrossRef] [Green Version]
- Roelofs, A. Annual Status Report 2008—Marine Aquarium Fish Fishery; Queensland Department of Primary Industries and Fisheries: Cairns, Australia, 2008. [Google Scholar]
- Brayford, H. Northern Territory Aquarium Species. A Guide to Vertebrate Species Harvested by the Northern Territory Aquarium Fishery; The Northern Territory Government, Department of Primary Industry, Fisheries and Mines: Darwin, Australia, 2008. [Google Scholar]
- Álvarez-Filip, L.; Gill, J.A.; Dulvy, N.K. Complex reef architecture supports more small-bodied fishes and longer food chains on Caribbean reefs. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Smith, T.; Hindell, J.S.; Jenkins, G.P.; Connolly, R.M.; Keough, M.J. Edge effects in patchy seagrass landscapes: The role of predation in determining fish distribution. J. Exp. Mar. Biol. Ecol. 2011, 399, 8–16. [Google Scholar] [CrossRef]
- Jenkins, G.P.; Hamer, P. Spatial variation in the use of seagrass and unvegetated habitats by post-settlement King George whiting (Percoidei: Sillaginidae) in relation to meiofaunal distribution and macrophyte structure. Mar. Ecol. Prog. Ser. 2001, 224, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Ford, J.; Williams, R.; Fowler, A.M.; Cox, D.R.; Suthers, I.M. Identifying critical estuarine seagrass habitat for settlement of coastally spawned fish. Mar. Ecol. Prog. Ser. 2010, 408, 181–193. [Google Scholar] [CrossRef]
- Jackson, E.; Rowden, A.; Attrill, M.; Bossey, S.J.; Malcolm, B. The importance of seagrass beds as a habitat for fishery species. Oceanogr. Mar. Biol. 2001, 39, 269–304. [Google Scholar]
- Connolly, R.M.; Hindell, J.S. Review of nekton patterns and ecological processes in seagrass landscapes. Estuar. Coast. Shelf Sci. 2006, 68, 433–444. [Google Scholar] [CrossRef]
- Gullström, M.; Berkström, C.; Öhman, M.C.; Bodin, M.; Dahlberg, M. Scale-dependent patterns of variability of a grazing parrotfish (Leptoscarus vaigiensis) in a tropical seagrass-dominated seascape. Mar. Biol. 2011, 158, 1483–1495. [Google Scholar] [CrossRef]
- Gullström, M.; Bodin, M.; Nilsson, P.; Öhman, M. Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar. Ecol. Prog. Ser. 2008, 363, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, A.; Hyndes, G.A. Patterns in the abundance and size-distribution of syngnathid fishes among habitats in a seagrass-dominated marine environment. Estuar. Coast. Shelf Sci. 2003, 57, 631–640. [Google Scholar] [CrossRef]



| Similarity | Group | Group Av. Sim. | Species | Sim/SD | Contrib.% | Cum.% |
| CPUE | Deep | 52.0 | Paramonacanthus japonicus | 4.6 | 26.5 | 26.5 |
| Siganus fuscescens | 1.7 | 22.4 | 48.9 | |||
| Lethrinus genivittatus | 3.8 | 18.8 | 67.8 | |||
| CPUE | Shallow | 47.9 | Secutor insidiator | 1.7 | 22.5 | 22.5 |
| Acetes spp. | 1.8 | 19.0 | 41.5 | |||
| Penaeus semisulcatus | 2.4 | 15.0 | 56.5 | |||
| BPUE | Deep | 46.9 | Paramonacanthus japonicus | 4.8 | 25.8 | 25.8 |
| Siganus fuscescens | 1.7 | 19.4 | 45.3 | |||
| Lethrinus genivittatus | 5.0 | 14.0 | 59.2 | |||
| BPUE | Shallow | 37.6 | Penaeus semisulcatus | 2.3 | 18.6 | 18.6 |
| Secutor insidiator | 1.5 | 15.2 | 33.8 | |||
| Dissimilarity | Group | Group Av. Diss. | Species | Diss/SD | Contrib.% | Cum.% |
| CPUE | Deep-Shallow | 75.5 | Secutor insidiator | 1.4 | 14.4 | 14.4 |
| Paramonacanthus japonicus | 2.9 | 9.9 | 24.2 | |||
| BPUE | Deep-Shallow | 79.9 | Paramonacanthus japonicus | 3.1 | 11.5 | 11.5 |
| Deep | Shallow | ||||||
|---|---|---|---|---|---|---|---|
| Commercial | Recreational | Aquarium | Commercial | Recreational | Aquarium | ||
| Number of species | Common | 4 | 1 | 4 | 7 | 3 | 2 |
| Rare | 14 | 11 | 9 | 15 | 13 | 8 | |
| Percentage of species | Common | 14.8% | 3.7% | 14.8% | 24.1% | 10.3% | 6.9% |
| Rare | 51.9% | 40.7% | 33.3% | 51.7% | 44.8% | 27.6% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, M.A.; McClure, E.C.; York, P.H.; Jinks, K.I.; Rasheed, M.A.; Sheaves, M.; Connolly, R.M. The Differential Importance of Deep and Shallow Seagrass to Nekton Assemblages of the Great Barrier Reef. Diversity 2020, 12, 292. https://0-doi-org.brum.beds.ac.uk/10.3390/d12080292
Hayes MA, McClure EC, York PH, Jinks KI, Rasheed MA, Sheaves M, Connolly RM. The Differential Importance of Deep and Shallow Seagrass to Nekton Assemblages of the Great Barrier Reef. Diversity. 2020; 12(8):292. https://0-doi-org.brum.beds.ac.uk/10.3390/d12080292
Chicago/Turabian StyleHayes, Matthew A., Eva C. McClure, Paul H. York, Kristin I. Jinks, Michael A. Rasheed, Marcus Sheaves, and Rod M. Connolly. 2020. "The Differential Importance of Deep and Shallow Seagrass to Nekton Assemblages of the Great Barrier Reef" Diversity 12, no. 8: 292. https://0-doi-org.brum.beds.ac.uk/10.3390/d12080292