Feeding Behavior, Shrinking, and the Role of Mucus in the Cannonball Jellyfish Stomolophus sp. 2 in Captivity
Abstract
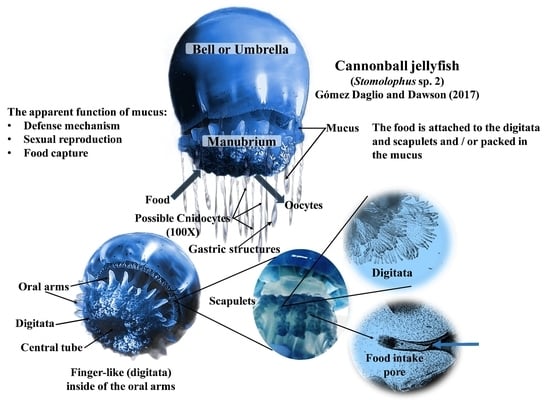
:1. Introduction
2. Materials and Methods
2.1. Feeding Essays
2.2. Mucus Samples
2.3. Reproduction
2.4. Change in Bell Diameter
3. Results
3.1. Feeding Behavior
3.2. Mucus Secretion
3.3. Reproduction
3.4. Change in Bell Diameter
4. Discussion
4.1. Feeding Behavior
4.2. Mucus Secretion
4.3. Size Reduction of Jellyfishes
4.4. Feeding Structures in Mucus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, C.E. Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia 2001, 451, 55–68. [Google Scholar] [CrossRef]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Peggy Hsieh, Y.H.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M.; et al. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish Biol. Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Brotz, L.; Pauly, D. Jellyfish populations in the Mediterranean Sea. Acta Adriat. 2012, 53, 211–230. [Google Scholar]
- Lynam, C.; Gibbons, M.; Axelsen, B.E.; Sparks, C.; Coetzee, J.; Heywood, B.; Brierley, A. Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 2006, 16, 492–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, M.N. A Functional Biology of Scyphozoa; Chapman & Hall: London, UK; Weinheim, Germany; New York, NY, USA; Tokyo, Japan; Melbourne, Australia; Madras, India, 1997; p. 316. [Google Scholar]
- Duarte, C.M.; Pitt, K.; Lucas, C.; Purcell, J.E.; Uye, S.-I.; Robinson, K.; Brotz, L.; Decker, M.B.; Sutherland, K.; Malej, A.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 91–97. [Google Scholar] [CrossRef]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef] [Green Version]
- Xian, W.; Kang, B.; Liu, R. Jellyfish Blooms in the Yangtze Estuary. Science 2005, 307, 41. [Google Scholar] [CrossRef] [Green Version]
- Amorim, K.; Mattmüller, R.; Algueró-Muñiz, M.; Meunier, C.; Alvarez-Fernandez, S.; Boersma, M.; Morais, P.; Teodósio Chícharo, M. Winter river discharge may affect summer estuarine jellyfish blooms. Mar. Ecol. Prog. Ser. 2018, 591, 253–265. [Google Scholar] [CrossRef]
- Brodeur, R.; Decker, M.; Ciannelli, L.; Purcell, J.; Bond, N.; Stabeno, P.; Acuna, E.; Hunt, G. Rise and fall of jellyfish in the eastern Bering Sea in relation to climate regime shifts. Prog. Oceanogr. 2008, 77, 103–111. [Google Scholar] [CrossRef]
- Purcell, J. Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Biol. Assoc. U. K. 2005, 85, 461–476. [Google Scholar] [CrossRef]
- Purcell, J. Jellyfish and Ctenophore Blooms Coincide with Human Proliferations and Environmental Perturbations. Annu. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Suchman, C.; Brodeur, R.; Daly, E.; Emmett, R. Large medusae in surface waters of the Northern California Current: Variability in relation to environmental conditions. Hydrobiologia 2012, 690, 113–125. [Google Scholar] [CrossRef]
- Boero, F.; Brotz, L.; Gibbons, M.; Piraino, S.; Zampardi, S. Impacts and effects of ocean warming on jellyfish. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; Laffoley, D., Baxter, J.M., Eds.; IUCN: Gland, Switzerland, 2016; pp. 213–237. [Google Scholar]
- Moller, H. Reduction of a Larval Herring Population by Jellyfish Predator. Science 1984, 224, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J. Predation on fish eggs and larvae by pelagic cnidarians and ctenophores. Bull. Mar. Sci. 1985, 37, 739–755. [Google Scholar]
- Purcell, J. Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia 2009, 616, 23–50. [Google Scholar] [CrossRef] [Green Version]
- Calder, D.R. Life History of the cannonball jellyfish, Stomolophus meleagris L. Agassiz, 1860 (Scyphozoa, Rhizostomida). Biol. Bull. 1982, 162, 149–162. [Google Scholar] [CrossRef]
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1228–1250. [Google Scholar] [CrossRef] [Green Version]
- Lucas, C.; Graham, W.; Widmer, C. Jellyfish Life histories: Role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol. 2012, 63, 133–196. [Google Scholar]
- Kikinger, R. Cotylorhiza tuberculata (Cnidaria: Scyphozoa)—Life History of a Stationary Population. Mar. Ecol. 2008, 13, 333–362. [Google Scholar] [CrossRef]
- Phillips, P.J.; Burke, W.D.; Keener, E.J. Observations on the Trophic Significance of Jellyfishes in Mississippi Sound with Quantitative Data on the Associative Behavior of Small Fishes with Medusae. Trans. Am. Fish. Soc. 1969, 98, 703–712. [Google Scholar] [CrossRef]
- Larson, R.J. Feeding in Coronate medusae (class Scyphoza, order Coronatae). Mar. Behav. Physiol. 1979, 6, 123–129. [Google Scholar] [CrossRef]
- Larson, R.J. A Note on the Feeding, Growth, and Reproduction of the Epipelagic Scyphomedusa Pelagia noctiluca (Forskål). Biol. Oceanogr. 1987, 4, 447–454. [Google Scholar]
- Nagata, R.M.; Morandini, A.C. Diet, prey selection, and individual feeding rates of the jellyfish Lychnorhiza lucerna (Scyphozoa, Rhizostomeae). Mar. Biol. 2018, 165, 1–17. [Google Scholar] [CrossRef]
- Hansson, L.J. Capture and digestion of the scyphozoan jellyfish Aurelia aurita by Cyanea capillata and prey response to predator contact. J. Plankton Res. 1997, 19, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Heeger, T.; Möller, H. Ultrastructural observations on prey capture and digestion in the scyphomedusa Aurelia aurita. Mar. Biol. 1987, 96, 391–400. [Google Scholar] [CrossRef]
- Lee, H.E.; Yoon, W.D.; Lim, D. Description of feeding apparatus and mechanism in Nemopilema nomurai Kishinouye (Scyphozoa: Rhizostomeae). Ocean Sci. J. 2008, 43, 61–65. [Google Scholar] [CrossRef]
- Fujita, S.; Kuranaga, E.; Nakajima, Y.-I. Cell proliferation controls body size growth, tentacle morphogenesis, and regeneration in hydrozoan jellyfish Cladonema pacificum. PeerJ 2019, 7, e7579. [Google Scholar] [CrossRef] [Green Version]
- Nagata, R.; Morandini, A.; Colin, S.; Migotto, A.; Costello, J. Transitions in morphologies, fluid regimes, and feeding mechanisms during development of the medusa Lychnorhiza lucerna. Mar. Ecol. Prog. Ser. 2016, 557, 145–159. [Google Scholar] [CrossRef]
- Larson, R.J. Diet, prey selection and daily ration of Stomolophus meleagris, a filter-feeding scyphomedusa from the NE Gulf of Mexico. Estuar. Coast. Shelf Sci. 1991, 32, 511–525. [Google Scholar] [CrossRef]
- Liu, W.; Mo, F.; Jiang, G.; Liang, H.; Ma, C.; Li, T.; Zhang, L.; Xiong, L.; Mariottini, G.; Zhang, J.; et al. Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea. Mar. Drugs 2018, 16, 341. [Google Scholar] [CrossRef] [Green Version]
- Patwa, A.; Thiéry, A.; Fabien, L.; Lilley, M.; Claire, B.; Bramard, J.-F.; Bottero, J.-Y.; Barthélémy, P. Accumulation of nanoparticles in “jellyfish” mucus: A bio-inspired route to decontamination of nano-waste. Sci. Rep. 2015, 5, 11387. [Google Scholar] [CrossRef] [Green Version]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection. NPJ Biofilms Microbiomes 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Shanks, A.; Graham, W.M. Chemical defense in a scyphomedusa. Mar. Ecol.-Prog. Ser. 1988, 45, 81–86. [Google Scholar] [CrossRef]
- Lewis Ames, C.; Klompen, A.; Badhiwala, K.; Muffett, K.; Reft, A.; Kumar, M.; Janssen, J.; Schultzhaus, J.; Field, L.; Muroski, M.; et al. Cassiosomes are stinging-cell structures in the mucus of the upside-down jellyfish Cassiopea xamachana. Commun. Biol. 2020, 3, 1–15. [Google Scholar]
- Lilley, M.; Elineau, A.; Ferraris, M.; Thiéry, A.; Stemmann, L.; Gorsky, G.; Fabien, L. Individual shrinking to enhance population survival: Quantifying the reproductive and metabolic expenditures of a starving jellyfish, Pelagia noctiluca. J. Plankton Res. 2014, 36, 1585–1597. [Google Scholar] [CrossRef] [Green Version]
- Kos Kramar, M.; Tinta, T.; Lucic, D.; Malej, A.; Turk, V. Bacteria associated with moon jellyfish during bloom and post-bloom periods in the Gulf of Trieste (northern Adriatic). PLoS ONE 2019, 14, e0198056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliff, S.M.; Wilczek, E.R.; Harris, R.J.; Bouldin, R.; Stoner, E.W. Evidence of microplastics from benthic jellyfish (Cassiopea xamachana) in Florida estuaries. Mar. Pollut. Bull. 2020, 159, 111521. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Daglio, L.; Dawson, M. Species richness of jellyfishes (Scyphozoa: Discomedusae) in the Tropical Eastern Pacific: Missed taxa, molecules, and morphology match in a biodiversity hotspot. Invertebr. Syst. 2017, 31, 635–663. [Google Scholar] [CrossRef]
- Carvalho Saucedo, L.; Martinez, J.; Garcia Dominguez, F.; Rodríguez-Jaramillo, M.; Padilla Serrato, J. Biología reproductiva de la medusa bola de cañón Stomolophus meleagris en la laguna Las Guásimas, Sonora, México. Hidrobiológica 2011, 21, 77–88. [Google Scholar]
- Purcell, J.E.; Fuentes, V.; Atienza, D.; Tilves, U.; Astorga, D.; Kawahara, M.; Hays, G.C. Use of respiration rates of scyphozoan jellyfish to estimate their effects on the food web. Hydrobiologia 2010, 645, 135–152. [Google Scholar] [CrossRef] [Green Version]
- Lechable, M.; Jan, A.; Weissbourd, B.; Uveira, J.; Gissat, L.; Collet, S.; Gilletta, L.; Chevalier, S.; Leclère, L.; Peron, S.; et al. An improved whole life cycle culture protocol for the hydrozoan genetic model Clytia hemisphaerica. Biol. Open 2020, 9, 1–13. [Google Scholar] [CrossRef]
- You, K.; Ma, C.; Gao, H.; Li, F.; Zhang, M.; Qiu, Y.; Wang, B. Research on the jellyfish (Rhopilema esculentum Kishinouye) and associated aquaculture techniques in China: Current status. Aquac. Int. 2007, 15, 479–488. [Google Scholar] [CrossRef]
- Guevara, M.; Lodeiros, C. Composición bioquímica de nauplios y metanauplios de Artemia sp. (Crustacea, Anostraca) proveniente de la salina artificial de Araya, nororiente de Venezuela. Cienc. Mar. 2003, 29, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Nava, M.F.; Alvarez-Borrego, S. Zooplankton in a whale shark (Rhincodon typus) feeding area of Bahía de los Angeles (Gulf of California). Hidrobiológica 2013, 23, 198–208. [Google Scholar]
- Sokal, R.; Rohlf, J.F. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman: New, York, NY, USA, 1995. [Google Scholar]
- Calder, D.R. Nematocysts of stages in the life cycle of Stomolophus meleagris, with keys to scyphistomae and ephyrae of western Atlantic Scyphozoa. Can. J. Zool. 1983, 61, 1185–1192. [Google Scholar] [CrossRef]
- López-Martínez, J.; Álvarez-Tello, J. The jellyfish fishery in Mexico. Agric. Sci. 2013, 4, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Bezio, N.; Costello, J.H.; Perry, E.; Colin, S. Effects of capture surface morphology on feeding success of scyphomedusae: A comparative study. Mar. Ecol. Prog. Ser. 2018, 596, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Costello, J.H.; Colin, S.P. Flow and feeding by swimming scyphomedusae. Mar. Biol. 1995, 124, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Bailey, K.M.; Batty, R.S. Laboratory study of predation by Aurelia aurita on larvae of cod, flounder, plaice and herring: Development and vulnerability to capture. Mar. Biol. 1984, 83, 287–291. [Google Scholar] [CrossRef]
- Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L. Metabolic diversity of microbial community associated with Rhizostoma pulmo (Scyphozoa: Rhizostomeae). J. Mar. Microbiol. 2017, 1, 5–8. [Google Scholar]
- Basso, L.; Rizzo, L.; Marzano, M.; Intranuovo, M.; Fosso, B.; Pesole, G.; Piraino, S.; Stabili, L. Jellyfish summer outbreaks as bacterial vectors and potential hazards for marine animals and humans’ health? The case of Rhizostoma pulmo (Scyphozoa, Cnidaria). Sci. Total Environ. 2019, 692, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.I.; Ohno, H.; Wada, N.; Ueno, S.; Goessler, W.; Kuehnelt, D.; Schlagenhaufen, C.; Kaise, T.; Irgolic, K.J. Occurrence of organo-arsenicals in jellyfishes and their mucus. Chemosphere 2001, 44, 743–749. [Google Scholar] [CrossRef]
- Pitt, K.A.; Welsh, D.T.; Condon, R.H. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia 2009, 616, 133–149. [Google Scholar] [CrossRef]
- Bythell, J.; Wild, C. Biology and ecology of coral mucus release. J. Exp. Mar. Biol. Ecol. 2011, 408, 88–93. [Google Scholar] [CrossRef]
- Rivera Ortega, J. Actividad Antimicrobiana del Mucus de Cnidarios Simbióticos. Master’s Thesis, National Autonomous University of Mexico, Puerto Morelos, Mexico, 2018. [Google Scholar]
- Rottini Sandrini, L.; Avian, M. Reproduction of Pelagia noctiluca in the central and northern Adriatic Sea. Hydrobiologia 1991, 216, 197–202. [Google Scholar] [CrossRef]
- Aglieri, G.; Papetti, C.; Zane, L.; Milisenda, G.; Boero, F.; Piraino, S. First evidence of inbreeding, relatedness and chaotic genetic patchiness in the holoplanktonic Jellyfish Pelagia noctiluca (Scyphozoa, Cnidaria). PLoS ONE 2014, 9, e99647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, H.; Båmstedt, U. Food regulation of growth and maturation in a natural population of Aurelia aurita (L.). J. Plankton Res. 1998, 20, 805–816. [Google Scholar] [CrossRef]
- Fu, Z.; Shibata, M.; Makabe, R.; Ikeda, H.; Uye, S.-I. Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita. Mar. Ecol. Prog. Ser. 2014, 510, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Lucas, C.H. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 2001, 451, 229–246. [Google Scholar] [CrossRef]
- Brewer, R.H. The Annual Pattern of Feeding, Growth, and Sexual Reproduction in Cyanea (Cnidaria: Scyphozoa) in the Niantic River Estuary, Connecticut. Biol. Bull. 1989, 176, 272–281. [Google Scholar] [CrossRef]
- Kremer, P. Effect of food availability on the metabolism of the ctenophore Mnemiopsis mccradyi. Mar. Biol. 1982, 71, 149–156. [Google Scholar] [CrossRef]
- Padilla-Serrato, J.G.; López-Martínez, J.; Acevedo-Cervantes, A.; Alcántara-Razo, E.; Rábago-Quiroz, C.H. Feeding of the scyphomedusa Stomolophus meleagris in the coastal lagoon Las Guásimas, northwest Mexico. Hidrobiológica 2013, 23, 218–226. [Google Scholar]
- Álvarez-Tello, F.J.; López-Martínez, J.; Lluch-Cota, D.B. Trophic spectrum and feeding pattern of cannonball jellyfish Stomolophus meleagris (Agassiz, 1862) from central Gulf of California. J. Mar. Biol. Assoc. U. K. 2016, 96, 1217–1227. [Google Scholar] [CrossRef]
- Carvalho-Saucedo, L.; García-Domínguez, F.; Rodríguez-Jaramillo, C.; López-Martínez, J. Variación lipídica en los ovocitos de la medusa Stomolophus meleagris (Scyphozoa: Rhizostomeae), durante el desarrollo gonádico, en la laguna Las Guásimas, Sonora, México. Rev. Biol. Trop. 2010, 58, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamner, W.; Jenssen, R. Growth, Degrowth, and Irreversible Cell Differentiation in Aurelia aurita. Integr. Comp. Biol. 1974, 14, 833–849. [Google Scholar]
- Raskoff, K.; Sommer, F.; Hamner, W.; Cross, K. Collection and Culture Techniques for Gelatinous Zooplankton. Biol. Bull. 2003, 204, 68–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niggl, W.; Naumann, M.S.; Struck, U.; Manasrah, R.; Wild, C. Organic matter release by the benthic upside-down jellyfish Cassiopea sp. fuels pelagic food webs in coral reefs. J. Exp. Mar. Biol. Ecol. 2010, 384, 99–106. [Google Scholar] [CrossRef]
- Tinta, T.; Klun, K.; Herndl, G.J. The importance of jellyfish–microbe interactions for biogeochemical cycles in the ocean. Limnol. Oceanogr. 2021, 66, 2011–2032. [Google Scholar] [CrossRef]
- Malej, A. Behaviour and trophic ecology of the jellyfish Pelagia noctiluca (Forsskål). J. Exp. Mar. Biol. Ecol. 1989, 126, 259–270. [Google Scholar] [CrossRef]
- Jordano, M.; Morandini, A.; Nagata, R. Is phenotypic plasticity determined by temperature and fluid regime in filter-feeding gelatinous organisms? J. Exp. Mar. Biol. Ecol. 2019, 522, 151238. [Google Scholar] [CrossRef]
- Frandsen, K.; Riisgård, H.U. Size dependent respiration and growth of jellyfish, Aurelia aurita. Sarsia 1997, 82, 307–312. [Google Scholar] [CrossRef]
- Tills, O.; Sun, X.-X.; Rundle, S.; Heimbach, T.; Gibson, T.; Cartwright, A.; Palmer, M.; Rudin-Bitterli, T.; Spicer, J. Reduced pH affects pulsing behaviour and body size in ephyrae of the moon jellyfish, Aurelia aurita. J. Exp. Mar. Biol. Ecol. 2016, 480, 54–61. [Google Scholar] [CrossRef]
- Aljbour, S.M.; Zimmer, M.; Kunzmann, A. Cellular respiration, oxygen consumption, and trade-offs of the jellyfish Cassiopea sp. in response to temperature change. J. Sea Res. 2017, 128, 92–97. [Google Scholar] [CrossRef]
- López-Martínez, J.; Arzola-Sotelo, E.A.; Nevárez-Martínez, M.O.; Álvarez-Tello, F.J.; Morales-Bojórquez, E. Modeling growth on the cannonball jellyfish Stomolophus meleagris based on a multi-model inference approach. Hydrobiologia 2020, 847, 1399–1422. [Google Scholar] [CrossRef]
- Dańko, A.; Schaible, R.; Dańko, M.J. Salinity Effects on Survival and Reproduction of Hydrozoan Eleutheria dichotoma. Estuaries Coasts 2020, 43, 360–374. [Google Scholar] [CrossRef] [Green Version]









| Date | Food Item | Items/7 L | Feeding Rate |
|---|---|---|---|
| 09–13 Feb | Artemia franciscana nauplii | 65,000 | 9.29/mL |
| 07–11 Mar | Cassostrea gigas “D” larvae | 65,000 | 9.29/mL |
| 19–23 Mar | Totoaba macdonaldi fish eggs | 2400 | 0.34/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Pacheco, A.V.; Gómez-Salinas, L.C.; Cisneros-Mata, M.Á.; Rodríguez-Félix, D.; Díaz-Tenorio, L.M.; Unzueta-Bustamante, M.L. Feeding Behavior, Shrinking, and the Role of Mucus in the Cannonball Jellyfish Stomolophus sp. 2 in Captivity. Diversity 2022, 14, 103. https://0-doi-org.brum.beds.ac.uk/10.3390/d14020103
Camacho-Pacheco AV, Gómez-Salinas LC, Cisneros-Mata MÁ, Rodríguez-Félix D, Díaz-Tenorio LM, Unzueta-Bustamante ML. Feeding Behavior, Shrinking, and the Role of Mucus in the Cannonball Jellyfish Stomolophus sp. 2 in Captivity. Diversity. 2022; 14(2):103. https://0-doi-org.brum.beds.ac.uk/10.3390/d14020103
Chicago/Turabian StyleCamacho-Pacheco, Alicia Verónica, Laura Cristina Gómez-Salinas, Miguel Ángel Cisneros-Mata, Demetrio Rodríguez-Félix, Lourdes Mariana Díaz-Tenorio, and Marco Linné Unzueta-Bustamante. 2022. "Feeding Behavior, Shrinking, and the Role of Mucus in the Cannonball Jellyfish Stomolophus sp. 2 in Captivity" Diversity 14, no. 2: 103. https://0-doi-org.brum.beds.ac.uk/10.3390/d14020103