Taxonomic Diversity of Decapod and Stomatopod Crustaceans Associated with Pocilloporid Corals in the Central Mexican Pacific
Abstract
:1. Introduction
2. Materials and Methods
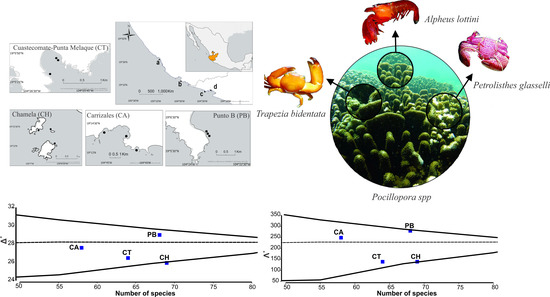
2.1. Study Area
2.2. Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stella, S.J.; Pratchett, M.S.; Hutchings, P.A.; Jones, G.P. Coral–associated invertebrates: Diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. Annu. Rev. 2011, 49, 43–104. [Google Scholar]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Counsell, C.W.; Donahue, M.J.; Edwards, K.F.; Franklin, E.C.; Hixon, M.A. Variation in coral-associated cryptofaunal communities across spatial scales and environmental gradients. Coral Reefs 2018, 37, 827–840. [Google Scholar] [CrossRef]
- Thiel, M.; Baeza, J.A. Factors affecting the social behaviour of crustaceans living symbiotically with other marine invertebrates: A modelling approach. Symbiosis 2001, 30, 163–190. [Google Scholar]
- Castro, P. Symbiotic Brachyura. In Treatise on Zoology-Anatomy, Taxonomy, Biology. The Crustacea Volume 9; Castro, P., Davie, P., Guinot, D., Schram, F.R., von Vaupel Klein, J.C., Eds.; Brill: Leiden, The Netherlands, 2015; pp. 543–581. [Google Scholar] [CrossRef]
- Abele, L.G. Comparative species composition and relative abundance of decapod crustaceans in marine habitats of Panama. Mar. Biol. 1976, 38, 263–278. [Google Scholar] [CrossRef]
- González-Gómez, R.; Briones-Fourzán, P.; Álvarez-Filip, L.; Lozano-Álvarez, E. Diversity and abundance of conspicuous macrocrustaceans on coral reefs differing in level of degradation. PeerJ 2018, 6, e4922. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 2006, 25, 609–615. [Google Scholar] [CrossRef]
- Head, C.E.; Bonsall, M.B.; Koldewey, H.; Pratchett, M.S.; Speight, M.; Rogers, A.D. High prevalence of obligate coral-dwelling decapods on dead corals in the Chagos Archipelago, central Indian Ocean. Coral Reefs 2015, 34, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Glynn, P.W. Defense by symbiotic crustacea of host corals elicited by chemical cues from predator. Oecologia 1980, 47, 287–290. [Google Scholar] [CrossRef] [PubMed]
- McKeon, C.S.; Moore, J.M. Species and size diversity in protective services offered by coral guard-crabs. PeerJ 2014, 2, e574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeon, C.S.; Stier, A.C.; McIlroy, S.E.; Bolker, B.M. Multiple defender effects: Synergistic coral defense by mutualist crustaceans. Oecologia 2012, 169, 1095–1103. [Google Scholar] [CrossRef]
- Britayev, T.A.; Mikheev, V. Clumped spatial distribution of scleractinian corals influences the structure of their symbiotic associations. Biol. Sci. 2013, 448, 45–48. [Google Scholar] [CrossRef]
- Randall, J.E. Food habits of reef fishes of the West Indies. Stud. Trap. Oceanogr. 1967, 5, 665–847. [Google Scholar]
- Enochs, I.C. Motile cryptofauna associated with live and dead coral substrates: Implications for coral mortality and framework erosion. Mar. Biol. 2012, 159, 709–722. [Google Scholar] [CrossRef]
- Leray, M.; Meyer, C.P.; Mills, S.C. Metabarcoding dietary analysis of coral dwelling predatory fish demonstrates the minor contribution of coral mutualists to their highly partitioned, generalist diet. PeerJ 2015, 3, e1047. [Google Scholar] [CrossRef]
- Reyes-Bonilla, H. Coral reefs of the Pacific coast of Mexico. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 331–349. [Google Scholar] [CrossRef]
- Hernández-Zulueta, J.; Rodríguez-Zaragoza, F.A.; Araya, R.; Vargas-Ponce, O.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, A.L.; Díaz-Pérez, L.; Ríos-Jara, E.; Ortiz, M. Multi-scale analysis of hermatypic coral assemblages at Mexican Central Pacific. Sci. Mar. 2017, 81, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Pereyra Ortega, R.T. Cangrejos Anomuros y Braquiuros (Crustacea: Decapoda) Simbiontes del Coral Pocillopora elegans de Los Islotes, Baja California Sur, Mexico. Undergraduate Thesis, Universidad Autónoma de Baja California Sur, La Paz, Mexico, 1998. [Google Scholar]
- Hernández, L. Camarones Stenopodideos y Carideos (Crustacea: Decapoda: Stenopodidae, Caridea) Asociados a Colonias de Coral, en Los Islotes, B.C.S., México. Undergraduate Thesis, Universidad Autónoma de Baja California Sur, La Paz, Mexico, 1999. [Google Scholar]
- Ramírez-Luna, S.; De La Cruz-Agüero, G.; Barrientos-Luján, N.A. Variación espacio-temporal de Porcellanidae, Majoidea y Xanthoidea asociados a corales del género Pocillopora en Bahías de Huatulco, México. In Contribuciones al Estudio de Los Crustáceos del Pacífico Este [Contributions to the Study of East Pacific Crustaceans]; Hendrickx, M.E., Ed.; Instituto de Ciencias del Mar y Limnología: Ciudad de México, México, 2002; pp. 233–254. [Google Scholar]
- Hernández, L.; Balart, E.F.; Reyes-Bonilla, H. Effect of hurricane John (2006) on the invertebrates associated with corals in Bahia de la Paz, Gulf of California. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008. [Google Scholar]
- Hernández, L.; Reyes-Bonilla, H.; Balart, E.F. Efecto del blanqueamiento del coral por baja temperatura en los crustáceos decápodos asociados a arrecifes del suroeste del Golfo de California. Rev. Mex. Biodivers. 2010, 81, 113–119. [Google Scholar] [CrossRef]
- Hernández, L.; Ramírez-Ortiz, G.; Reyes-Bonilla, H. Coral–associated decapods (Crustacea) from the Mexican Tropical Pacific coast. Zootaxa 2013, 3609, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Ayón-Parente, M.; Hermoso-Salazar, M.; Hendrickx, M.E.; Galván-Villa, C.M.; Ríos-Jara, E.; Bastida-Izaguirre, D. The caridean shrimps (Crustacea: Decapoda: Caridea: Alpheoidea, Palaemonoidea, and Processoidea) from Bahía Chamela, Mexico. Rev. Mex. Bio. 2016, 87, 311–327. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Warwick, R.M. The taxonomic distinctness measure of biodiversity: Weighting of step lengths between hierarchical levels. Mar. Ecol. Prog. Ser. 1999, 184, 21–29. [Google Scholar] [CrossRef]
- Magurran, E.A. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; p. 248. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, X.; Liu, C.; Xu, Z.; Wang, J.; Qian, S.; Zhou, H. Response of traditional and taxonomic distinctness diversity indices of benthic macroinvertebrates to environmental degradation gradient in a large Chinese shallow lake. Environ. Sci. Pollut. Res. 2020, 27, 21804–21815. [Google Scholar] [CrossRef]
- Leonard, D.R.P.; Clarke, K.R.; Somerfield, P.J.; Warwick, R.M. The application of an indicator based on taxonomic distinctness for UK marine biodiversity assessment. J. Environ. Manag. 2006, 78, 52–62. [Google Scholar] [CrossRef]
- Roger, S.I.; Clarke, K.R.; Reynolds, J.D. The taxonomic distinctness of coastal bottom-dwelling fish communities of the North-east Atlantic. J. Anim. Ecol. 1999, 68, 769–792. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, B.; Sun, Z.; Cao, L.; Lu, Y. Application of taxonomic distinctness indices of fish assemblages for assessing effects of river-lake disconnection and eutrophication in floodplain lakes. Ecol. Indic. 2020, 110, 105955. [Google Scholar] [CrossRef]
- Heino, J.; Soininen, J.; Lappalainen, J.; Virtanen, R. The relationship between species richness and taxonomic distinctness in freshwater organisms. Limnol. Oceanogr. 2005, 50, 978–986. [Google Scholar] [CrossRef]
- Jiang, X.; Song, Z.; Xiong, J.; Xie, Z. Can excluding non-insect taxa from stream macroinvertebrate surveys enhance the sensitivity of taxonomic distinctness indices to human disturbance? Ecol. Indic. 2014, 41, 175–182. [Google Scholar] [CrossRef]
- Heino, J.; Mykrä, H.; Hämäläinen, H.; Aroviita, J.; Muotka, T. Responses of taxonomic distinctness and species diversity indices to anthropogenic impacts and natural environmental gradients in stream macroinvertebrates. Freshw. Biol. 2007, 52, 1846–1861. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Fattorini, S. Phylogenetic diversity of regional beetle faunas at high latitudes: Patterns, drivers and chance along ecological gradients. Biodivers. Conserv. 2015, 24, 2751–2767. [Google Scholar] [CrossRef]
- López-Pérez, A.; Granja-Fernández, R.; Benítez-Villalobos, F.; Jiménez-Antonio, O. Pocillopora damicornis-associated echinoderm fauna: Richness and community structure across the southern Mexican Pacific. Marine Biodiversity 2017, 47, 481–490. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Kessler, W.S. The circulation of the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 181–217. [Google Scholar] [CrossRef]
- Fiedler, P.C.; Talley, L.D. Hydrography of the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 143–180. [Google Scholar] [CrossRef]
- Fiedler, P.C.; Lavín, M.F. Oceanographic conditions of the eastern tropical Pacific. In Coral Reefs of the Eastern Tropical Pacific, 1st ed.; Glynn, P.W., Manzello, D.O., Enochs, I.C., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 1, pp. 59–83. [Google Scholar]
- Rathbun, M.J. The cancroid crabs of America of the families Euryalidae, Portunidae, Atelecyclidae, Cancridae, and Xanthidae. Bull. U. S. Natl. Mus. 1930, 152, 1–609. [Google Scholar] [CrossRef]
- Haig, J. The Porcellanidae (Crustacea Anomura) of the eastern Pacific. In Allan Hancock Pacific Expedition; University of Southern California: Los Angeles, CA, USA, 1960; Volume 24, pp. 1–440. [Google Scholar]
- Abele, L.G.; Kim, W. An Illustrated Guide to the Marine Decapod Crustaceans of Florida Volume 8; Technical Series; Department of Environmental Regulations, Florida State University: Tallahassee, FL, USA, 1986. [Google Scholar]
- Castro, P. Eastern Pacific species of Trapezia (Crustacea, Brachyura: Trapeziidae), sibling species symbiotic with reef corals. Bull. Mar. Sci. 1996, 58, 531–554. [Google Scholar]
- Anker, A.; Ahyong, S.T.; Noël, P.Y.; Palmer, A.R. Morphological phylogeny of alpheid shrimps: Parallel preadaptation and the origin of a key morphological innovation, the snapping claw. Evolution 2006, 60, 2507–2528. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, M.E.; Ayón-Parente, M.; Félix-Pico, E.; Vargas-López, G. Hermit crabs (Crustacea: Paguroidea) in the biological collection of CICIMAR, Instituto Politécnico Nacional, La Paz, Baja California Sur, México. In Contribution to the Study of East Pacific Crustaceans; Hendrickx, M.E., Ed.; Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México: Ciudad de México, México, 2008; Volume 5, pp. 17–21. [Google Scholar]
- Ayón-Parente, M. Taxonomía, Zoogeografía y Aspectos Ecológicos de los Cangrejos Ermitaños de la Familia Diogenidae (Crustacea: Decapoda: Anomura) del Pacífico Mexicano. Ph.D. Thesis, Posgrado en Ciencias del Mar y Limnología, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Ciudad de México, México, 2009. [Google Scholar]
- García-Madrigal, M.S.; Andréu-Sánchez, L.I. Los cangrejos porcelánidos (Decapoda: Anomura) del Pacífico sur de México, incluyendo una lista y clave de identificación para todas las especies del Pacífico oriental tropical. Ciencia y Mar 2009, 13, 23–54. [Google Scholar]
- Hermoso-Salazar, A.M. Sistemática y Biogeografía del género Synalpheus (Decapoda: Caridea) del Pacífico Oriental. Ph.D. Thesis, Universidad Nacional Autónoma de México, Mexico City, México, 2009. [Google Scholar]
- Salgado-Barragán, J.; Hendrickx, M.E. Clave ilustrada para la identificación de los estomatópodos (Crustacea: Hoplocarida) del Pacífico oriental. Rev. Mex. Biodivers. 2010, 81, 1–49. [Google Scholar] [CrossRef]
- Hiller, A.; Lessios, H.A. Marine species formation along the rise of Central America: The anomuran crab Megalobrachium. Mol. Ecol. 2020, 29, 413–428. [Google Scholar] [CrossRef]
- González-Oreja, J.; De la Fuente-Díaz-Ordaz, A.A.; Hernández-Santín, L.; Buzo-Franco, D.; Bonache-Regidor, C. Evaluación de estimadores no paramétricos de la riqueza de especies. Un ejemplo con aves en áreas verdes de la ciudad de Puebla, México. Anim. Biodivers. Conserv. 2010, 33, 31–45. [Google Scholar]
- Collwell, R. Estimate S: Statistical Estimation of Species Richness and Shared Species from Samples. 2015. Available online: viceroy.colorado.edu/estimates/EstimateSPages/EstimateSRegistration.htm (accessed on 9 January 2020).
- Warwick, R.M.; Clarke, K.R. New biodiversity measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Gorley, R.N. Primer v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- de Gier, W.; Becker, C. A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations. Diversity 2020, 12, 431. [Google Scholar] [CrossRef]
- Barrientos-Lujan, N.A.; Rodríguez-Zaragoza, F.A.; López-Pérez, A. Richness, abundance and spatial heterogeneity of gastropods and bivalves in coral ecosystems across the Mexican Tropical Pacific. J. Molluscan Stud. 2021, 87, eyab004. [Google Scholar] [CrossRef]
- Holthuis, L.B. A General Revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas; University of Southern California: Los Angeles, CA, USA, 1951; Volume 11, pp. 1–332. [Google Scholar]
- Fransen, C.H. Taxonomy, phylogeny, historical biogeography, and historical ecology of the genus Pontonia (Crustacea: Decapoda: Caridea: Palaemonidae). Zool. Verh. 2002, 336, 1–433. [Google Scholar]
- Britayev, T.A.; Spiridonov, V.A.; Deart, Y.V.; El-Sherbiny, M. Biodiversity of the community associated with Pocillopora verrucosa (Scleractinia: Pocilloporidae) in the Red Sea. Mar. Bio. 2017, 47, 1093–1109. [Google Scholar] [CrossRef]
- Coles, S. Species diversity of decapods associated with living and dead reef coral Pocillopora meandrina. Mar. Ecol. Prog. Ser. 1980, 2, 281–291. [Google Scholar] [CrossRef]
- Stella, J.S.; Jones, G.P.; Pratchett, M.S. Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs 2010, 29, 957–973. [Google Scholar] [CrossRef]
- Abele, L.G.W.; Patton, K. The size of coral heads and the community biology of associated decapod crustaceans. J. Biogeogra. 1976, 3, 35–47. [Google Scholar] [CrossRef]
- Alvarado, J.J.; Vargas-Castillo, R. Invertebrados asociados al coral constructor de arrecifes Pocillopora damicornis en Playa Blanca, Bahía Culebra, Costa Rica. Rev. Biol. Trop. 2012, 60, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Canizales-Flores, H.M.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A.; Cupul-Magaña, A.L. A Long-term symbiotic relationship: Recruitment and fidelity of the crab Trapezia on its coral host Pocillopora. Diversity 2021, 13, 450. [Google Scholar] [CrossRef]
- Castro, P. Movements between coral colonies in Trapezia ferruginea (Crustacea: Brachyura), an obligate symbiont of scleractinian corals. Mar. Biol. 1978, 46, 237–245. [Google Scholar] [CrossRef]
- Bhat, A.; Magurran, A.E. Taxonomic distinctness in a linear system: A test using a tropical freshwater fish assemblage. Ecography 2006, 29, 104–110. [Google Scholar] [CrossRef]
- Leira, M.; Chen, G.; Dalton, C.; Irvine, K.; Taylor, D. Patterns in freshwater diatom taxonomic distinctness along an eutrophication gradient. Freshw. Biol. 2009, 54, 1–14. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Fraschetti, S.; Musco, L.; Guarnieri, G.; Terlizzi, A. Low sensi-tiveness of taxonomic distinctness indices to human impacts: Evidences acrossmarine benthic organisms and habitat types. Ecol. Indic. 2011, 11, 448–455. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Domínguez, A.; Ayón-Parente, M.; Hendrickx, M.E.; Ríos-Jara, E.; Vargas-Ponce, O.; Esqueda-González, M.d.C.; Rodríguez-Zaragoza, F.A. Taxonomic Diversity of Decapod and Stomatopod Crustaceans Associated with Pocilloporid Corals in the Central Mexican Pacific. Diversity 2022, 14, 72. https://0-doi-org.brum.beds.ac.uk/10.3390/d14020072
Alonso-Domínguez A, Ayón-Parente M, Hendrickx ME, Ríos-Jara E, Vargas-Ponce O, Esqueda-González MdC, Rodríguez-Zaragoza FA. Taxonomic Diversity of Decapod and Stomatopod Crustaceans Associated with Pocilloporid Corals in the Central Mexican Pacific. Diversity. 2022; 14(2):72. https://0-doi-org.brum.beds.ac.uk/10.3390/d14020072
Chicago/Turabian StyleAlonso-Domínguez, Arizbeth, Manuel Ayón-Parente, Michel E. Hendrickx, Eduardo Ríos-Jara, Ofelia Vargas-Ponce, María del Carmen Esqueda-González, and Fabián Alejandro Rodríguez-Zaragoza. 2022. "Taxonomic Diversity of Decapod and Stomatopod Crustaceans Associated with Pocilloporid Corals in the Central Mexican Pacific" Diversity 14, no. 2: 72. https://0-doi-org.brum.beds.ac.uk/10.3390/d14020072