Diversity of Nearctic Dragonflies and Damselflies (Odonata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definition of Nearctic Realm
2.2. Species Occurrence Data
2.3. Functional Traits and Conservation Status
2.4. Species Distribution Modeling
2.5. Calculating Richness and Endemism
3. Results
3.1. Richness and Corrected Weighted Endemism (CWE)
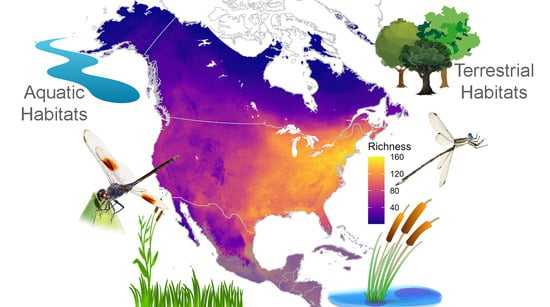
3.2. Richness and Endemism by Terrestrial and Aquatic Habitats
3.3. Richness of Species According to IUCN Red List Category
3.4. Sampling Effort
4. Discussion
4.1. General Diversity Patterns
4.2. Are There Differences in Diversity Patterns Shown by Forest and Non-Forest Species?
4.3. Are There Differences in Diversity Patterns Shown by Lotic and Lentic Species?
4.4. Are There Areas with Relatively High Endemism?
4.5. Are There Areas with a Relatively High Percentage of Globally Threatened Species?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagen, H.A. Synopsis of the Odonata of America. Proc. Boston Soc. Nat. Hist. 1875, 18, 20–96. [Google Scholar]
- Needham, J.G.; Westfall, M.J.; May, M. Dragonflies of North America: The Odonata (Anisoptera) Fauna of Canada, the Continental United States, Northern Mexico and the Greater Antilles; Scientific Publishers: Singapore, 2014. [Google Scholar]
- Davis, J. Dragon Flies: The Fascinating World of Odonata in Art, Culture and Religion; 2011. [Google Scholar]
- Hillerman, T. The Boy Who Made Dragonfly, 2nd ed.; University of New Mexico Press: Albuquerque, NM, USA, 1986. [Google Scholar]
- Fincke, O.M. Sperm Competition in the Damselfly Enallagma Hageni Walsh (Odonata: Coenagrionidae): Benefits of Multiple Mating to Males and Females. Behav. Ecol. Sociobiol. 1984, 14, 235–240. [Google Scholar] [CrossRef]
- Garrison, R. A Synopsis of the Genus Argia of the United States with Keys and Descriptions of New Species, Argia sabino, A. leonorae, and A. pima (Odonata: Coenagrionidae). Trans. Am. Entomol. Soc. 1994, 120, 287–368. [Google Scholar]
- May, M. Simultaneous Control of Head and Thoracic Temperature by the Green Darner Dragonfly Anax junius (Odonata: Aeshnidae). J. Exp. Biol. 1995, 198, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.; May, M.; Kjer, K. Phylogeny of the Higher Libelluloidea (Anisoptera: Odonata): An Exploration of the Most Speciose Superfamily of Dragonflies. Mol. Phylogenet. Evol. 2007, 45, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, S.W. Dragonflies through Binoculars: A Field Guide to Dragonflies of North America; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tennessen, K.J. Dragonfly Nymphs of North America: An Identification Guide; Springer: Berlin, Germany, 2019. [Google Scholar]
- Upson, S.; Danforth, D.; Gonzalez-Soriano, E.; Behrstock, R.A.; Bailowitz, R.A. A Preliminary Checklist of the Odonata of Sonora, Mexico. Bull. Am. Odonatol. 2007, 10, 23–51. [Google Scholar]
- Cuevas-Yañez, K. Los Odonatos (Insecta: Odonata) de La Hidroeléctrica de Patla (El Pozo) y del Río Tecpatlán, Zihuateutla, Puebla, México. Dugesiana 2007, 14, 83–91. [Google Scholar]
- Escoto-Moreno, J.A.; Novelo-Gutiérrez, R.; Sigala-Rodríguez, J.; Escoto-Rocha, J.; Carrillo-Lara, D.E.; Reynoso-Velasco, D. First Records of Odonata from Zacatecas State, Mexico. Not. Odonatol. 2015, 8, 151–155. [Google Scholar]
- Novelo-Gutiérrez, R.; Gonzalez-Soriano, E. Odonata de La Reserva de La Biosfera La Michilia, Durango, Mexico. Parte II. Nayades. Folia Entomol. Mex. 1991, 164, 107–164. [Google Scholar]
- Ortega-Salas, H.; Gonzalez-Soriano, E. Odonata of the Cuatro Ciénegas Basin. In Animal Diversity and Biogeography of the Cuatro Ciénegas Basin; Álvarez, F., Ojeda, M., Eds.; Springer: Berlin, Germany, 2019; pp. 117–128. ISBN 978-3-030-11261-5. [Google Scholar]
- Cuevas–Yañez, K.; Espinosa–Rivera, J.C.; Martínez–Falcón, A.P.; Córdoba–Aguilar, A. Are All Mexican Odonate Species Documented? An Assessment of Species Richness. Syst. Biodivers. 2017, 15, 253–258. [Google Scholar] [CrossRef]
- Escalante, T.; Morrone, J.J. Toward a Biogeographic Regionalization of the Nearctic Region: Area Nomenclature and Digital Map. Zootaxa 2021, 5027, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Halffter, G.; Morrone, J.J. An Analytical Review of Halffter’s Mexican Transition Zone, and Its Relevance for Evolutionary Biogeography, Ecology and Biogeographical Regionalization. Zootaxa 2017, 4226, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. The Mexican Transition Zone; Springer: Cham, Switzerland, 2020; ISBN 9783030479169. [Google Scholar]
- Paulson, D.R.; Dunkle, S.W. A Checklist of North American Odonata: Including English Name, Etymology, Type Locality, and Distribution. 2012 Edition. Occas. Pap. Slater Museum Nat. Hist. 2021, 56, 1–86. [Google Scholar]
- Bailowitz, R.; Danforth, D.; Upson, S. A Field Guide to the Dragonflies & Damselflies of Arizona and Sonora; Nova Granada Publications: Tucson, AZ, USA, 2015. [Google Scholar]
- Paulson, D.R. Dragonflies and Damselflies of the West; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Paulson, D.R. Dragonflies and Damselflies of the East.; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- González-Soriano, E.; Novelo-Gutiérrez, R. Odonata. In Biodiversidad, Taxonomia y Biogeografia de Artropodos de Mexico: Hacia una Sintesis de Su Conocimiento; Llorente-Bousquets, J., Garcia-Aldrete, A.N., González-Soriano, E., Eds.; Facultad de Ciencias UNAM: México City, Mexico, 1996; pp. 147–167. [Google Scholar]
- González-Soriano, E.; Novelo-Gutiérrez, R. Odonata of Mexico revisited. In Odonata: Biology of Dragonflies; Tyagi, B.K., Ed.; Scientific Publishers: Jodhpur, India, 2007; pp. 105–136. [Google Scholar]
- Abbott, J.C. Odonata Central. Available online: http://www.odonatacentral.org (accessed on 3 February 2022).
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 2 March 2022).
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. Bioscience 2001, 51, 933. [Google Scholar] [CrossRef]
- Behrstock, R.A.; Danforth, D.; Upson, S. A List of the Odonata of Chihuahua State, Mexico, Including New State Records and the First Mexican Record of Argia Alberta, Kennedy, 1918. Bull. Am. Odonatol. 2007, 10, 52–63. [Google Scholar]
- Calvert, P.P. Odonata. In Biologia Centrali Americana: Insecta Neuroptera; R. Porter & Dulau Co.: London, UK, 1892–1908; pp. 17–420. [Google Scholar]
- Calvert, P.P. Contributions to a Knowledge of the Odonata of the Neotropical Region, Exclusive of Mexico and Central America. Ann. Carnegie Museum 1909, 6, 73–280. [Google Scholar]
- González-Soriano, E.; Noguera, F.A.; Zaragoza-Caballero, S.; Ramírez-García, E. Odonata de un Bosque Tropical Caducifolio: Sierra de San Javier, Sonora, México. Rev. Mex. Biodivers. 2009, 80, 341–348. [Google Scholar] [CrossRef]
- Gonzalez-Soriano, E.; Novelo-Gutiérrez, R. Odonata de La Reserva de La Biosfera de La Michilia, Durango, Mexico. Parte I. Imagos. Folia Entomol. Mex. 1991, 105, 67–105. [Google Scholar]
- Escoto-Moreno, J.A.; Márquez, J.; Novelo-Gutiérrez, R. Los Odonatos (Insecta: Odonata) Del Estado de Hidalgo, México: Situación Actual y Perspectivas. Rev. Mex. Biodivers. 2014, 85, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Google Earth Pro 2021.
- Earl, C.; Belitz, M.W.; Laffan, S.W.; Barve, V.; Barve, N.; Soltis, D.E.; Allen, J.M.; Soltis, P.S.; Mishler, B.D.; Kawahara, A.Y.; et al. Spatial Phylogenetics of Butterflies in Relation to Environmental Drivers and Angiosperm Diversity across North America. iScience 2021, 24, 102239. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized Cleaning of Occurrence Records from Biological Collection Databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Certain, G.; Kraan, C.; Dormann, C.F. Stacking Species Distribution Models and Adjusting Bias by Linking Them to Macroecological Models. Glob. Ecol. Biogeogr. 2014, 23, 99–112. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Hof, C.; Brändle, M.; Brandl, R. Lentic Odonates Have Larger and More Northern Ranges than Lotic Species. J. Biogeogr. 2006, 33, 63–70. [Google Scholar] [CrossRef]
- Corbet, P.S. Forests as habitats for dragonflies (Odonata). In Forests and Dragonflies; Cordero-Rivera, A., Ed.; Pensoft: Sofia, Bulgaria; Moscow, Russia, 2006; pp. 13–35. [Google Scholar]
- Sahlén, G. Specialists vs. generalists among dragonflies—The importance of forest environments in the formation of diverse species pools. In Forests and Dragonflies; Cordero-Rivera, A., Ed.; Pensoft: Sofia, Bulgaria; Moscow, Russia, 2006; pp. 153–179. [Google Scholar]
- Bota-Sierra, C.A.; García-Robledo, C.; Escobar, F.; Novelo-Gutiérrez, R.; Londoño, G.A. Environment, Taxonomy and Morphology Constrain Insect Thermal Physiology along Tropical Mountains. Funct. Ecol. 2022. early view. [Google Scholar] [CrossRef]
- Davis Rabosky, A.R.; Cox, C.L.; Rabosky, D.L.; Title, P.O.; Holmes, I.A.; Feldman, A.; McGuire, J.A. Coral Snakes Predict the Evolution of Mimicry across New World Snakes. Nat. Commun. 2016, 7, 11484. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The Crucial Role of the Accessible Area in Ecological Niche Modeling and Species Distribution Modeling. Ecol. Modell. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Steen, V.A.; Elphick, C.S.; Tingley, M.W. An Evaluation of Stringent Filtering to Improve Species Distribution Models from Citizen Science Data. Divers. Distrib. 2019, 25, 1857–1869. [Google Scholar] [CrossRef]
- Hijmans, R.J. Package ‘Raster’—Geographic Data Analysis and Modeling; CRAN Repos, 2019. Available online: https://rspatial.org/raster (accessed on 10 May 2022).
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling; R Package Version 1.1-4; Cran. 2017. Available online: https://cran.r-project.org/web/packages/dismo/dismo.pdf (accessed on 10 May 2022).
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Laffan, S.W.; Miller, J.T. Spatial Distribution of Species Richness and Endemism of the Genus Acacia in Australia. Aust. J. Bot. 2011, 59, 601–609. [Google Scholar] [CrossRef]
- Segers, H. Global Diversity of Rotifers (Rotifera) in Freshwater. Hydrobiologia 2008, 595, 49–59. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Clausnitzer, V.; Dijkstra, K.D.B.; Orr, A.G.; Paulson, D.R.; Van Tol, J. Global Diversity of Dragonflies (Odonata) in Freshwater. Hydrobiologia 2008, 595, 351–363. [Google Scholar] [CrossRef]
- Lévêque, C.; Oberdorff, T.; Paugy, D.; Stiassny, M.L.J.; Tedesco, P.A. Global Diversity of Fish (Pisces) in Freshwater. Hydrobiologia 2008, 595, 545–567. [Google Scholar] [CrossRef]
- Väinölä, R.; Witt, J.D.S.; Grabowski, M.; Bradbury, J.H.; Jazdzewski, K.; Sket, B. Global Diversity of Amphipods (Amphipoda; Crustacea) in Freshwater. Hydrobiologia 2008, 595, 241–255. [Google Scholar] [CrossRef]
- Balian, E.V.; Segers, H.; Lévèque, C.; Martens, K. The Freshwater Animal Diversity Assessment: An Overview of the Results. Hydrobiologia 2008, 595, 627–637. [Google Scholar] [CrossRef]
- Bogan, A.E. Global Diversity of Freshwater Mussels (Mollusca, Bivalvia) in Freshwater. Hydrobiologia 2008, 595, 139–147. [Google Scholar] [CrossRef]
- Bour, R. Global Diversity of Turtles (Chelonii; Reptilia) in Freshwater. Hydrobiologia 2008, 595, 593–598. [Google Scholar] [CrossRef]
- Crandall, K.A.; Buhay, J.E. Global Diversity of Crayfish (Astacidae, Cambaridae, and Parastacidae—Decapoda) in Freshwater. Hydrobiologia 2008, 595, 295–301. [Google Scholar] [CrossRef]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global Diversity of Gastropods (Gastropoda; Mollusca) in Freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Batzer, D.P.; Baldwin, A.H. Wetland Habitats of North America: Ecology and Conservation Concerns; University of California Press: Berkley, CA, USA, 2012. [Google Scholar]
- Rey, J.R.; Walton, W.E.; Wolfe, R.J.; Roxanne, C.; O’Connell, S.M.; Berg, J.; Sakolsky-Hoopes, G.E.; Laderman, A.D. North American Wetlands and Mosquito Control. Int. J. Environ. Res. Public Health 2012, 9, 4537–4605. [Google Scholar] [CrossRef]
- Stewart, K.W.; Stark, B.P. Nymphs of North American Stonefly Genera (Plecoptera); The Caddis Press: Columbus, OH, USA, 2002. [Google Scholar]
- Fochetti, R.; Tierno De Figueroa, J.M. Global Diversity of Stoneflies (Plecoptera; Insecta) in Freshwater. Hydrobiologia 2008, 595, 365–377. [Google Scholar] [CrossRef]
- De Moor, F.C.; Ivanov, V.D. Global Diversity of Caddisflies (Trichoptera: Insecta) in Freshwater. Hydrobiologia 2008, 595, 393–407. [Google Scholar] [CrossRef]
- Williams, P.H. Mapping Variations in the Strength and Breadth of Biogeographic Transition Zones Using Species Turnover. Proc. R. Soc. B Biol. Sci. 1996, 263, 579–588. [Google Scholar] [CrossRef]
- Mishler, B.D.; Guralnick, R.; Soltis, P.S.; Smith, S.A.; Soltis, D.E.; Barve, N.; Allen, J.M.; Laffan, S.W. Spatial Phylogenetics of the North American Flora. J. Syst. Evol. 2020, 58, 393–405. [Google Scholar] [CrossRef]
- Collins, S.D.; Abbott, J.C.; McIntyre, N.E. Quantifying the Degree of Bias from Using County-Scale Data in Species Distribution Modeling: Can Increasing Sample Size or Using County-Averaged Environmental Data Reduce Distributional Overprediction? Ecol. Evol. 2017, 7, 6012–6022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botella, C.; Joly, A.; Monestiez, P.; Bonnet, P.; Munoz, F. Bias in Presence-Only Niche Models Related to Sampling Effort and Species Niches: Lessons for Background Point Selection. PLoS ONE 2020, 15, e0232078. [Google Scholar] [CrossRef]
- Ortega-Salas, H.; González-Soriano, E. A New Species of Libellula Linnaeus, 1758, from the Cuatro Ciénegas Basin, Coahuila, México (Anisoptera: Libellulidae). Zootaxa 2015, 4028, 589–594. [Google Scholar] [CrossRef]
- Vega-Sánchez, Y.M.; Mendoza-Cuenca, L.F.; González-Rodríguez, A. Hetaerina Calverti (Odonata: Zygoptera: Calopterygidae) sp. Nov., a New Cryptic Species of the American Rubyspot Complex. Zootaxa 2020, 4766, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, V.J.; Boudot, J.-P.; Futahashi, R.; Abbott, J.C.; Bota-Sierra, C.A.; Guralnick, R.; Bybee, S.M.; Ware, J.L.; Belitz, M.W. Diversity of Palaearctic Dragonflies and Damselflies (Odonata). Diversity 2022. in preparation. [Google Scholar]
- Barber-James, H.M.; Gattolliat, J.L.; Sartori, M.; Hubbard, M.D. Global Diversity of Mayflies (Ephemeroptera, Insecta) in Freshwater. Hydrobiologia 2008, 595, 339–350. [Google Scholar] [CrossRef]
- Suhling, F.; Schenk, K.; Padeffke, T.; Martens, A. A Field Study of Larval Development in a Dragonfly Assemblage in African Desert Ponds (Odonata). Hydrobiologia 2004, 528, 75–85. [Google Scholar] [CrossRef]
- Van Damme, K.; Dumont, H.J. A Drought-Resistant Larva of Pantala flavescens (Fabricius, 1798) (Odonata: Libellulidae) in the Lençóis Maranhenses, NE-Brazil. Int. J. Odonatol. 1999, 2, 69–76. [Google Scholar] [CrossRef]
- Ware, J.; Kohli, M.K.; Mendoza, C.M.; Troast, D.; Jinguji, H.; Hobson, K.A.; Sahlén, G.; Anderson, R.C.; Suhling, F. Evidence for Widespread Gene Flow and Migration in the Globe Skimmer Dragonfly Pantala Flavescens. Int. J. Odonatol. 2022, 25, 43–55. [Google Scholar] [CrossRef]
- Paulson, D.R. An Asiatic Dragonfly, Crocothemis servilia (Drury), Established in Florida (Anisoptera: Libellulidae). Not. Odonatol. 1978, 1, 9–10. [Google Scholar]
- Buczyński, P.; Bielak-Bielecki, P. Crocothemis servilia (Drury, 1773) (Odonata: Libellulidae) Introduced with Aquarium Plants to Lublin (Poland). Ann. UMCS Biol. 2012, 67, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Cordero Rivera, A.; Lorenzo Carballa, M.O.; Utzeri, C.; Vieira, V. Parthenogenetic Ischnura hastata (Say), Widespread in the Azores (Zygoptera: Coenagrionidae). Odonatologica 2005, 34, 1–9. [Google Scholar]
- Lorenzo-Carballa, M.O.; Beatty, C.D.; Utzeri, C.; Vieira, V.; Cordero-Rivera, A. Parthenogenetic Ischnura Hastata Revisited: Present Status and Notes on Population Ecology and Behaviour (Odonata: Coenagrionidae). Int. J. Odonatol. 2009, 12, 395–411. [Google Scholar] [CrossRef]
- Bota-Sierra, C.A.; Flórez-V, C.; Escobar, F.; Sandoval-H, J.; Novelo-Gutiérrez, R.; Londoño, G.A.; Cordero-Rivera, A. The Importance of Tropical Mountain Forests for the Conservation of Dragonfly Biodiversity: A Case from the Colombian Western Andes. Int. J. Odonatol. 2021, 24, 233–247. [Google Scholar] [CrossRef]
- Bogan, M.T.; Noriega-Felix, N.; Vidal-Aguilar, S.L.; Findley, L.T.; Lytle, D.A.; Gutiérrez-Ruacho, O.G.; Alvarado-Castro, J.A.; Varela-Romero, A. Biogeography and Conservation of Aquatic Fauna in Spring-Fed Tropical Canyons of the Southern Sonoran Desert, Mexico. Biodivers. Conserv. 2014, 23, 2705–2748. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Tognelli, M.F.; Lasso, C.A.; Bota-Sierra, C.A.; Jiménez-Segura, L.F.; Cox, N.A. Estado de Conservación y Distribución de la Biodiversidad de Agua Dulce en Los Andes Tropicales, 1st ed.; Tognelli, M.F., Lasso, C.A., Bota-Sierra, C.A., Jiménez-Segura, L.F., Cox, N.A., Eds.; IUCN: Gland, Switzerland; Cambridge, UK; Arlington, TX, USA, 2016; ISBN 9782831717913. [Google Scholar]
- González-Soriano, E. Leptobasis melinogaster spec. nov., a New Species from Mexico (Zygoptera: Coenagrionidae). Odonatologica 2002, 31, 181–185. [Google Scholar]








| Bioclimatic Variable | Description | Mean Permutation Contribution |
|---|---|---|
| Bio 8 | Mean temperature of wettest quarter | 14.4 |
| Bio 2 | Mean diurnal range | 13.7 |
| Bio 1 | Annual mean temperature | 11.1 |
| Bio 4 | Temperature seasonality | 10.3 |
| Bio 15 | Precipitation seasonality | 10.2 |
| Bio 9 | Mean temperature of driest quarter | 9.6 |
| Bio 5 | Max temperature of warmest month | 6.7 |
| Bio 13 | Precipitation of wettest month | 5.4 |
| Bio 14 | Precipitation of driest month | 5.0 |
| Bio 12 | Annual precipitation | 3.7 |
| Bio 6 | Min temperature of coldest month | 3.45 |
| Bio 16 | Precipitation of wettest quarter | 3.2 |
| Bio 17 | Precipitation of driest quarter | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbott, J.C.; Bota-Sierra, C.A.; Guralnick, R.; Kalkman, V.; González-Soriano, E.; Novelo-Gutiérrez, R.; Bybee, S.; Ware, J.; Belitz, M.W. Diversity of Nearctic Dragonflies and Damselflies (Odonata). Diversity 2022, 14, 575. https://0-doi-org.brum.beds.ac.uk/10.3390/d14070575
Abbott JC, Bota-Sierra CA, Guralnick R, Kalkman V, González-Soriano E, Novelo-Gutiérrez R, Bybee S, Ware J, Belitz MW. Diversity of Nearctic Dragonflies and Damselflies (Odonata). Diversity. 2022; 14(7):575. https://0-doi-org.brum.beds.ac.uk/10.3390/d14070575
Chicago/Turabian StyleAbbott, John C., Cornelio A. Bota-Sierra, Robert Guralnick, Vincent Kalkman, Enrique González-Soriano, Rodolfo Novelo-Gutiérrez, Seth Bybee, Jessica Ware, and Michael W. Belitz. 2022. "Diversity of Nearctic Dragonflies and Damselflies (Odonata)" Diversity 14, no. 7: 575. https://0-doi-org.brum.beds.ac.uk/10.3390/d14070575