Nanoparticles as Emerging Labels in Electrochemical Immunosensors
Abstract
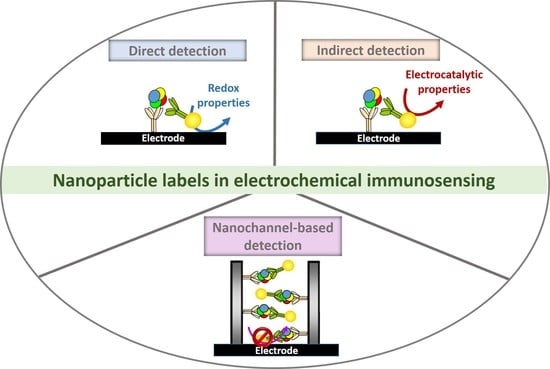
:1. Introduction
2. Nanoparticles as Electroactive Labels
2.1. Gold Nanoparticles (AuNPs)
2.2. Silver Nanoparticles (AgNPs)
2.3. Quantum Dots (QDs)
2.4. Other Nanoparticles (NPs)
2.4.1. Cerium Oxide Nanoparticles (CeO2 NPs)
2.4.2. Mercury Selenide Nanoparticles (HgSe NPs)
2.4.3. Copper-Based Nanoparticles (CuNPs)
3. Nanoparticles as Electrocatalytic Labels
3.1. Gold Nanoparticles (AuNPs)
3.1.1. Silver Electrodeposition
3.1.2. Hydrogen Evolution Reaction (HER)
3.1.3. H2O2 Reduction
3.2. Silver-Based Nanoparticles (AgNPs)
3.3. Platinum-Based Nanoparticles (PtNPs)
3.4. Palladium-Based Nanoparticles (PdNPs)
3.5. Multimetallic NPs
3.5.1. Gold-Based Multimetallic Nanoparticles
3.5.2. Palladium–Platinum-Based Bimetallic Nanoparticles
3.6. Iridium-Based Nanoparticles (IrNPs)
3.7. Ferromagnetic Nanoparticles
3.8. Molybdenum Disulphide Nanoparticles (MoS2 NPs)
4. Nanoparticles as Blocking Agents in Nanochannels-Based Immunosensors
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-CN | 4-chloro-1-naphthol |
| AFP | Alpha-fetoprotein |
| AgNPs | Silver nanoparticles |
| anti-tTG IgA | Anti-tissue transglutaminase immunoglobulin A antibodies |
| anti-tTG IgG | Anti-tissue transglutaminase immunoglobulin G antibodies |
| ApoE | Apolipoprotein E |
| ASV | Anodic stripping voltammetry |
| AuNPs | Gold nanoparticles |
| BSA-OP | Phosphorylated bovine serum albumin |
| CEA | Carcinoembryonic antigen |
| CeO2 NPs | Cerium oxide nanoparticles |
| CuNPs | Copper nanoparticles |
| CTCs | Circulating tumour cells |
| CV | Cyclic voltammetry |
| CVs | Cyclic voltammograms |
| DPV | Differential pulse voltammetry |
| E. coli | Escherichia coli |
| Fe3O4 NPs | Ferromagnetic nanoparticles |
| GCE | Glassy carbon electrode |
| GSH | Glutathione |
| HBeAg | Hepatitis B e antigen |
| HBsAg | Hepatitis B surface antigen |
| HER | Hydrogen evolution reaction |
| HgSe NPs | Mercury selenide nanoparticles |
| HIgG | Human Immunoglobulin G |
| HRP | Horseradish peroxidase |
| IgG | Immunoglobulin G |
| IrNPs | Iridium nanoparticles |
| IrO2 NPs | Iridium oxide nanoparticles |
| LSV | Linear sweep voltammetry |
| MBs | Magnetic beads |
| MoS2 NPs | Molybdenum disulphide nanoparticles |
| NP(s) | Nanoparticle(s) |
| OP-BChE | Organophosphorylated butyrylcholinesterase |
| ORR | Oxygen reduction reaction |
| PBDE | Polybrominated diphenyl ether |
| PBS | Sodium phosphate buffer |
| PdNPs | Palladium nanoparticles |
| POC | Point-of-care |
| PVP | Polyvinyl pyrrolidone |
| PTHrP | Parathyroid hormone-related protein |
| PtNPs | Platinum nanoparticles |
| PSA | Prostate specific antigen |
| QDs | Quantum Dots |
| SC | Sodium citrate |
| SCC-Ag | Squamous cell carcinoma antigen |
| SPEs | Screen-printed electrodes |
| ssDNA | Single strand DNA |
| STX | Saxitoxin |
| STEM | Scanning transmission electron microscope |
| SWV | Square wave voltammetry |
| TBBPA-DHEE | Tetrabromobisphenol A bis(2-hydroxyethyl) ether |
| TMB | 3,3’,5,5’-Tetramethylbenzidine |
| UPD | Under potential deposition |
| WOR | Water oxidation reaction |
References
- De la Escosura-Muñiz, A.; Ambrosi, A.; Merkoçi, A. Electrochemical analysis with nanoparticle-based biosystems. TrAC Trends Anal. Chem. 2008, 27, 568–584. [Google Scholar] [CrossRef]
- Zhou, F.; Yao, Y.; Luo, J.; Zhang, X.; Zhang, Y.; Yin, D.; Gao, F.; Wang, P. Proximity hybridization-regulated catalytic DNA hairpin assembly for electrochemical immunoassay based on in situ DNA template-synthesized Pd nanoparticles. Anal. Chim. Acta 2017, 969, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Fojta, M.; Daňhel, A.; Havran, L.; Vyskočil, V. Recent progress in electrochemical sensors and assays for DNA damage and repair. TrAC Trends Anal. Chem. 2016, 79, 160–167. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, B.; Yang, R.; Liu, J. Filling in the Gaps between Nanozymes and Enzymes: Challenges and Opportunities. Bioconj. Chem. 2017, 28, 2903–2909. [Google Scholar] [CrossRef]
- Singh, S. Cerium oxide based nanozymes: Redox phenomenon at biointerfaces. Biointerphases 2016, 11, 04B202. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; He, X.; Zhang, Z.; Zhao, Y. Ceria Nanoparticles as Enzyme Mimetics. Chin. J. Chem. 2017, 35, 791–800. [Google Scholar] [CrossRef]
- De La Escosura-Muñiz, A.; Parolo, C.; Merkoi, A. Immunosensing using nanoparticles. Mater. Today 2010, 13, 24–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-based electrochemical immunosensors. Micromachines 2019, 10, 397. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Mohammadi, S.; Salimi, A. Current advances of carbon dots based biosensors for tumor marker detection, cancer cells analysis and bioimaging. TrAC Trends Anal. Chem. 2019, 115, 83–99. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Nanomaterial Labels in Electrochemical Immunosensors and Immunoassays. Talanta 2007, 74, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Castañeda, M.T.; de la Escosura-Muñiz, A.; Mekoçi, A. Gold nanoparticles a powerful label for affinity electrochemical biosensors. Biosens. Using Nanomater. 2009, 6, 177–197. [Google Scholar]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Dequaire, M.; Degrand, C.; Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. A renewable electrochemical magnetic immunosensor based on gold nanoparticle labels. J. Nanosci. Nanotechnol. 2005, 5, 1060–1065. [Google Scholar] [CrossRef]
- De Oliveira, T.R.; Martucci, D.H.; Faria, R.C. Simple disposable microfluidic device for Salmonella typhimurium detection by magneto-immunoassay. Sens. Actuators B Chem. 2018, 255, 684–691. [Google Scholar] [CrossRef]
- Xu, T.; Jia, X.; Chen, X.; Ma, Z. Simultaneous electrochemical detection of multiple tumor markers using metal ions tagged immunocolloidal gold. Biosens. Bioelectron. 2014, 56, 174–179. [Google Scholar] [CrossRef]
- González García, M.B.; Costa García, A. Adsorptive stripping voltammetric behaviour of colloidal gold and immunogold on carbon paste electrode. Bioelectrochem. Bioenergy 1995, 38, 389–395. [Google Scholar] [CrossRef]
- Ambrosi, A.; Castañeda, M.T.; Killard, A.J.; Smyth, M.R.; Alegret, S.; Merkoçi, A. Double-codified gold nanolabels for enhanced immunoanalysis. Anal. Chem. 2007, 79, 5232–5240. [Google Scholar] [CrossRef] [PubMed]
- De La Escosura-Muñiz, A.; Parolo, C.; Maran, F.; Merkoçi, A. Size-dependent direct electrochemical detection of gold nanoparticles: Application in magnetoimmunoassays. Nanoscale 2011, 3, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Tschulik, K.; Batchelor-Mcauley, C.; Toh, H.-S.; Stuart, E.J.E.; Compton, R.G. Electrochemical studies of silver nanoparticles: A guide for experimentalists and a perspective. Phys. Chem. Chem. Phys. 2014, 16, 616–623. [Google Scholar] [CrossRef]
- Ting, B.P.; Zhang, J.; Khan, M.; Yang, Y.Y.; Ying, J.Y. The solid-state Ag/AgCl process as a highly sensitive detection mechanism for an electrochemical immunosensor. Chem. Commun. 2009, 6231–6233. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Han, K.; Sun, H.; Yin, J.; Zhao, J.; Wang, B.; Tang, Y. Melamine functionalized silver nanoparticles as the probe for electrochemical sensing of clenbuterol. ACS Appl. Mater. Interfaces 2014, 6, 8667–8672. [Google Scholar] [CrossRef]
- Khristunova, Y.; Korotkova, E.; Kratochvil, B.; Barek, J.; Dorozhko, E.; Vyskocil, V.; Plotnikov, E.; Voronova, O.; Sidelnikov, V. Preparation and Investigation of Silver Nanoparticle—Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis. Sensors 2019, 19, 2103. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, J.; Ruditskiy, A.; Liu, M.; Xia, Y. Oxidative etching and its role in manipulating the nucleation and growth of noble-metal nanocrystals. Chem. Mater. 2014, 26, 22–33. [Google Scholar] [CrossRef]
- Russo, L.; Puntes, V.; Merkoçi, A. Tunable electrochemistry of gold-silver alloy nanoshells. Nano Res. 2018, 11, 6336–6345. [Google Scholar] [CrossRef]
- Russo, L.; Leva Bueno, J.; Bergua, J.F.; Costantini, M.; Giannetto, M.; Puntes, V.; de La Escosura-Muñiz, A.; Merkoçi, A. Low-Cost Strategy for the Development of a Rapid Electrochemical Assay for Bacteria Detection Based on AuAg Nanoshells. ACS Omega 2018, 3, 18849–18856. [Google Scholar] [CrossRef]
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in 3D microscopic semiconductor crystals. Jept Lett. 1982, 34, 345–348. [Google Scholar]
- Nanocrystals in their prime. Nat. Nanotechnol. 2014, 9, 325. [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Ding, Z.; Myung, N. Electrochemistry and electrogenerated chemiluminescence of semiconductor nanocrystals in solutions and in films. Struct. Bond 2005, 118, 1–57. [Google Scholar]
- Grabolle, M.; Ziegler, J.; Merkulov, A.; Nann, T.; Resch-Genger, U. Stability and fluorescence quantum yield of CdSe-ZnS quantum dots—Influence of the thickness of the ZnS shell. Ann. N. Y. Acad. Sci. 2008, 1130, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Montoro Bustos, A.R.; Trapiella-Alfonso, L.; Encinar, J.R.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. Elemental and molecular detection for Quantum Dots-based immunoassays: A critical appraisal. Biosens. Bioelectron. 2012, 33, 165–171. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Polsky, R.; Merkoçi, A. Electrochemical stripping detection of DNA hybridization based on cadmium sulfide nanoparticle tags. Electrochem. Commun. 2002, 4, 722–726. [Google Scholar] [CrossRef]
- Yang, M.; Javadi, A.; Gong, S. Sensitive electrochemical immunosensor for the detection of cancer biomarker using quantum dot functionalized graphene sheets as labels. Sens. Actuators B Chem. 2011, 155, 357–360. [Google Scholar] [CrossRef]
- Lu, D.; Wang, J.; Wang, L.; Du, D.; Timchalk, C.; Barry, R.; Lin, Y. A novel nanoparticle-based disposable electrochemical immunosensor for diagnosis of exposure to toxic organophosphorus agents. Adv. Funct. Mater. 2011, 21, 4371–4378. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Biosensor array based on the in situ detection of quantum dots as electrochemical label. Sens. Actuators B Chem. 2013, 182, 184–189. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Electrochemical immunosensor for anti-tissue transglutaminase antibodies based on the in situ detection of quantum dots. Talanta 2014, 130, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; Costa-García, A. Towards a blocking-free electrochemical immunosensing strategy for anti-transglutaminase antibodies using screen-printed electrodes. Bioelectrochemistry 2015, 105, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Pinwattana, K.; Wang, J.; Lin, C.T.; Wu, H.; Du, D.; Lin, Y.; Chailapakul, O. CdSe/ZnS quantum dots based electrochemical immunoassay for the detection of phosphorylated bovine serum albumin. Biosens. Bioelectron. 2010, 26, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A.; Marcolino-Junior, L.H.; Marín, S.; Fatibello-Filho, O.; Alegret, S. Detection of cadmium sulphide nanoparticles by using screen-printed electrodes and a handheld device. Nanotechnology 2007, 18, 035502–035507. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Morales-Narváez, E.; Merkoçi, A. On-chip magneto-immunoassay for Alzheimer’s biomarker electrochemical detection by using quantum dots as labels. Biosens. Bioelectron. 2014, 54, 279–284. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Cadevall, M.; Merkoçi, A. Enhanced detection of quantum dots labeled protein by simultaneous bismuth electrodeposition into microfluidic channel. Electrophoresis 2016, 37, 432–437. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Andreescu, D.; Bulbul, G.; Özel, R.E.; Hayat, A.; Sardesai, N.; Andreescu, S. Applications and implications of nanoceria reactivity: Measurement tools and environmental impact. Environ. Sci. Nano 2014, 1, 445–458. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Ispas, C.; Njagi, J.; Cates, M.; Andreescu, S. Electrochemical Studies of Ceria as Electrode Material for Sensing and Biosensing Applications. J. Electrochem. Soc. 2008, 155, F169. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Vanegas, D.C.; Taguchi, M.; Burrs, S.L.; Sharma, P.; McLamore, E.S. A nanoceria-platinum-graphene nanocomposite for electrochemical biosensing. Biosens. Bioelectron. 2014, 58, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xue, Q.; Jiao, C.; Liu, H.; Zhou, Y.; Ma, H.; Yang, Q. A non-enzymatic nanoceria electrode for non-invasive glucose monitoring. Anal. Methods 2018, 10, 2151–2159. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Fernández-Murillo, L.; García-Alonso, F.J.; de la Escosura-Muñiz, A.; Costa-García, A. Nanoceria quantification based on its oxidative effect towards the ferrocyanide/ferricyanide system. J. Electroanal. Chem. 2019, 840, 338–342. [Google Scholar] [CrossRef]
- Mathe, M.K.; Cox, S.M.; Venkatasamy, V.; Happek, U.; Stickney, J.L. Formation of HgSe Thin Films Using Electrochemical Atomic Layer Epitaxy. J. Electrochem. Soc. 2005, 152, C751–C755. [Google Scholar] [CrossRef]
- Mattson, G.; Nyholm, L.; Olin, A. Cathodic stripping voltammetry of HgSe. J. Electroanal. Chem. 1994, 377, 149–162. [Google Scholar] [CrossRef]
- Venkatasamy, V.; Mathe, M.K.; Cox, S.M.; Happek, U.; Stickney, J.L. Optimization studies of HgSe thin film deposition by electrochemical atomic layer epitaxy (EC-ALE). Electrochim. Acta 2006, 51, 4347–4351. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Wang, F. Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Wang, F. Reversible dissolution of glutathione-mediated HgSexS 1-x nanoparticles and possible significance in Hg-Se antagonism. Chem. Res. Toxicol. 2009, 22, 1827–1832. [Google Scholar] [CrossRef]
- Bouzas-Ramos, D.; Menéndez-Miranda, M.; Costa-Fernández, J.M.; Encinar, J.R.; Sanz-Medel, A. Precise determination of the nanoparticle concentration and ligand density of engineered water-soluble HgSe fluorescent nanoparticles. RSC Adv. 2016, 6, 19964–19972. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Bouzas-Ramos, D.; Encinar, J.R.; Costa-Fernández, J.M.; de la Escosura-Muñiz, A.; Costa-García, A. Simple and rapid electrochemical quantification of water-stabilized HgSe nanoparticles of great concern in environmental studies. Talanta 2019, 200, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Wang, L.; Zhu, J.; Jiang, W. Cascade Signal Amplification Based on Copper Nanoparticle-Reported Rolling Circle Amplification for Ultrasensitive Electrochemical Detection of the Prostate Cancer Biomarker. ACS Appl. Mater. Interfaces 2016, 8, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Chen, J.; Chen, X. Alizarin red S/copper ion-based ensemble for fluorescence turn on detection of glutathione with tunable dynamic range. Biosens. Bioelectron. 2012, 38, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, P.; Mao, X.; Yin, Y.; Cao, Y. Sensitive detection of glutathione by using DNA-templated copper nanoparticles as electrochemical reporters. Sens. Actuators B Chem. 2017, 238, 325–330. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Tang, Y.; Miao, P. DNA-templated copper nanoparticles for voltammetric analysis of endonuclease activity. Analyst 2018, 143, 1685–1690. [Google Scholar] [CrossRef]
- Qing, Z.; Bai, A.; Xing, S.; Zou, Z.; He, X.; Wang, K.; Yang, R. Progress in biosensor based on DNA-templated copper nanoparticles. Biosens. Bioelectron. 2019, 137, 96–109. [Google Scholar] [CrossRef]
- Rotaru, A.; Dutta, S.; Jentzsch, E.; Gothelf, K.; Mokhir, A. Selective dsDNA-templated formation of copper nanoparticles in solution. Angew. Chem. Int. Ed. 2010, 49, 5665–5667. [Google Scholar] [CrossRef]
- Xu, F.; Shi, H.; He, X.; Wang, K.; He, D.; Guo, Q.; Qing, Z.; Yan, L.; Ye, X.; Li, D.; et al. Concatemeric dsDNA-templated copper nanoparticles strategy with improved sensitivity and stability based on rolling circle replication and its application in microRNA detection. Anal. Chem. 2014, 86, 6976–6982. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhao, L.; Bao, T.; Wen, W.; Zhang, X.; Wang, S. Integrated amplified aptasensor with in-situ precise preparation of copper nanoclusters for ultrasensitive electrochemical detection of microRNA 21. Biosens. Bioelectron. 2017, 98, 386–391. [Google Scholar] [CrossRef]
- Miao, P.; Zhang, T.; Xu, J.; Tang, Y. Electrochemical Detection of miRNA Combining T7 Exonuclease-Assisted Cascade Signal Amplification and DNA-Templated Copper Nanoparticles. Anal. Chem. 2018, 90, 11154–11160. [Google Scholar] [CrossRef]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ye, Y.; Liu, S. Gold nanoparticle-based signal amplification for biosensing. Anal. Biochem. 2011, 417, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Maltez-da Costa, M.; Merkoçi, A. Controlling the electrochemical deposition of silver onto gold nanoparticles: Reducing interferences and increasing the sensitivity of magnetoimmuno assays. Biosens. Bioelectron. 2009, 24, 2475–2482. [Google Scholar] [CrossRef]
- Lai, G.; Wang, L.; Wu, J.; Ju, H.; Yan, F. Electrochemical stripping analysis of nanogold label-induced silver deposition for ultrasensitive multiplexed detection of tumor markers. Anal. Chim. Acta 2012, 721, 1–6. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, J.; Ju, H.; Yan, F. Multiplexed electrochemical immunoassay using streptavidin/nanogold/carbon nanohorn as a signal tag to induce silver deposition. Anal. Chim. Acta 2014, 847, 37–43. [Google Scholar] [CrossRef]
- Chikae, M.; Idegami, K.; Kerman, K.; Nagatani, N.; Ishikawa, M.; Takamura, Y.; Tamiya, E. Direct fabrication of catalytic metal nanoparticles onto the surface of a screen-printed carbon electrode. Electrochem. Commun. 2006, 8, 1375–1380. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Sánchez-Espinel, C.; Díaz-Freitas, B.; González-Fernández, Á.; Maltez-Da Costa, M.; Merkoçi, A. Rapid identification and quantification of tumor cells using an electrocatalytic method based on gold nanoparticles. Anal. Chem. 2009, 81, 10268–10274. [Google Scholar] [CrossRef]
- Maltez-da Costa, M.; de la Escosura-Muñiz, A.; Nogués, C.; Barrios, L.; Ibáñez, E.; Merkoçi, A. Detection of circulating cancer cells using electrocatalytic gold nanoparticles. Small 2012, 8, 3605–3612. [Google Scholar] [CrossRef]
- Maltez-da Costa, M.; de la Escosura-Muñiz, A.; Nogués, C.; Barrios, L. Simple Monitoring of Cancer Cells Using Nanoparticles. Nano Lett. 2012, 12, 4164–4171. [Google Scholar] [CrossRef]
- Maltez-da Costa, M.; de la Escosura-Muñiz, A.; Merkoçi, A. Electrochemical quantification of gold nanoparticles based on their catalytic properties toward hydrogen formation: Application in magnetoimmunoassays. Electrochem. Commun. 2010, 12, 1501–1504. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Maltez-Da Costa, M.; Sánchez-Espinel, C.; Díaz-Freitas, B.; Fernández-Suarez, J.; González-Fernández, Á.; Merkoçi, A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010, 26, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Plichta, Z.; Horák, D.; Merkoçi, A. Alzheimer’s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosens. Bioelectron. 2015, 67, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.-R.H.A.-A.; de la Escosura-Muñiz, A.; Merkoçi, A. Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens. Bioelectron. 2015, 67, 511–515. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Baptista-Pires, L.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Magnetic Bead/Gold Nanoparticle Double-Labeled Primers for Electrochemical Detection of Isothermal Amplified Leishmania DNA. Small 2015, 12, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Jirkovský, J.S.; Halasa, M.; Schiffrin, D.J. Kinetics of electrocatalytic reduction of oxygen and hydrogen peroxide on dispersed gold nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 8042–8052. [Google Scholar] [CrossRef]
- Jia, H.; Gao, P.; Ma, H.; Li, Y.; Gao, J.; Du, B.; Wei, Q. Ultrasensitive electrochemical immunosensor for squamous cell carcinoma antigen detection using lamellar montmorillonite-gold nanostructures as signal amplification. Talanta 2015, 132, 803–808. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Gyimah, E.; Zhu, N.; Wang, K.; Wu, X.; Zhang, Z. A novel electrochemical immunosensor based on catalase functionalized AuNPs-loaded self-assembled polymer nanospheres for ultrasensitive detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether. Anal. Chim. Acta 2019, 1048, 50–57. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Jia, Y.; Li, Y.; Wang, P.; Liu, Q.; Xu, Z.; Li, X. Sandwich-type electrochemical immunosensor for sensitive detection of CEA based on the enhanced effects of Ag NPs@CS spaced Hemin/rGO. Biosens. Bioelectron. 2019, 126, 785–791. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Feng, J.; Gao, Z.; Lv, H.; Ren, X.; Wei, Q. Facile synthesis of MoS2@Cu2O-Pt nanohybrid as enzyme-mimetic label for the detection of the Hepatitis B surface antigen. Biosens. Bioelectron. 2018, 100, 512–518. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Li, M.; Li, F.; Han, J.; Dong, Y.; Chen, Z. A novel sandwich-type electrochemical immunosensor for PSA detection based on PtCu bimetallic hybrid (2D/2D) rGO/ g-C3N4. Biosens. Bioelectron. 2017, 91, 441–448. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Li, P.; Zheng, J. A novel electrochemical immunosensor based on nonenzymatic Ag@Au-Fe3O4 nanoelectrocatalyst for protein biomarker detection. Biosens. Bioelectron. 2016, 85, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Polsky, R.; Gill, R.; Kaganovsky, L.; Willner, I. Nucleic Acid-Functionalized Pt Nanoparticles: Catalytic Labels for the Amplified Electrochemical Detection of Biomolecules. Anal. Chem. 2006, 78, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Pei, F.; Wang, P.; Ma, E.; Yang, Q.; Yu, H.; Gao, C.; Li, Y.; Liu, Q.; Dong, Y. A sandwich-type electrochemical immunosensor based on RhPt NDs/NH2-GS and Au NPs/PPy NS for quantitative detection hepatitis B surface antigen. Bioelectrochemistry 2019, 126, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tian, L.; Zhao, G.; Huang, Y.; Wei, Q.; Cao, W. Ultrasensitive electrochemical immunosensor for alpha fetoprotein detection based on platinum nanoparticles anchored on cobalt oxide/graphene nanosheets for signal amplification. Anal. Chim. Acta 2017, 986, 138–144. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Li, N.; Zhang, Y.; Yan, T.; Ma, H.; Wei, Q. Sandwich-type electrochemical immunosensor for the detection of AFP based on Pd octahedral and APTES-M-CeO2-GS as signal labels. Biosens. Bioelectron. 2016, 79, 482–487. [Google Scholar] [CrossRef]
- Dai, L.; Li, Y.; Wang, Y.; Luo, X.; Wei, D.; Feng, R.; Yan, T. A prostate-specific antigen electrochemical immunosensor based on Pd NPs functionalized electroactive Co-MOF signal amplification strategy. Biosens. Bioelectron. 2019, 132, 97–104. [Google Scholar] [CrossRef]
- Jin, X.; Zhong, Y.; Chen, L.; Xu, L.; Wu, Y.; Fu, F.F. A Palladium-Doped Graphitic Carbon Nitride Nanosheet with High Peroxidase-Like Activity: Preparation, Characterization, and Application in Glucose Detection. Part. Part. Syst. Charact. 2018, 35, 1700359. [Google Scholar] [CrossRef]
- Jin, X.; Chen, J.; Zeng, X.; Xu, L.; Wu, Y.; Fu, F. A signal-on magnetic electrochemical immunosensor for ultra-sensitive detection of saxitoxin using palladium-doped graphitic carbon nitride-based non-competitive strategy. Biosens. Bioelectron. 2019, 128, 45–51. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448–466. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, C.; Ko, S.M.; Nam, J.M. Metal alloy hybrid nanoparticles with enhanced catalytic activities in fuel cell applications. J. Solid State Chem. 2019, 270, 295–303. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Su, Y.; Li, F.; Ma, H.; Li, H.; Du, B.; Wei, Q. Ultrasensitive non-mediator electrochemical immunosensors using Au/Ag/Au core/double shell nanoparticles as enzyme-mimetic labels. Talanta 2014, 124, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Li, F.; Feng, J.; Li, M.; Chen, L.; Dong, Y. Ultrasensitive electrochemical immunosensor for quantitative detection of SCCA using Co3O4@CeO2-Au@Pt nanocomposite as enzyme-mimetic labels. Biosens. Bioelectron. 2017, 92, 33–39. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Q.; Liu, Q.; Li, Y.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu2O nanoparticles for prostate specific antigen detection. Biosens. Bioelectron. 2018, 99, 450–457. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Lv, H.; Feng, J.; Gao, Z.; Wang, P.; Dong, Y. Sandwich-type electrochemical immunosensor based on Au@Ag supported on functionalized phenolic resin microporous carbon spheres for ultrasensitive analysis of α-fetoprotein. Biosens. Bioelectron. 2018, 106, 142–148. [Google Scholar] [CrossRef]
- Zhu, F.; Zhao, G.; Dou, W. Electrochemical sandwich immunoassay for Escherichia coli O157:H7 based on the use of magnetic nanoparticles and graphene functionalized with electrocatalytically active Au@ Pt core/shell nanoparticles. Microchim. Acta 2018, 185, 455. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Zhang, C.; Zhang, S.; Dong, Y. Enhanced peroxidase-like properties of Au@Pt DNs/NG/Cu2+ and application of sandwich-type electrochemical immunosensor for highly sensitive detection of CEA. Biosens. Bioelectron. 2018, 112, 1–7. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Zhang, X.; Feng, J.; Kong, L.; Wang, P.; Chen, Z.; Dong, Y.; Wei, Q. Ultrasensitive electrochemical immunosensor for quantitative detection of HBeAg using Au@Pd/MoS2@MWCNTs nanocomposite as enzyme-mimetic labels. Biosens. Bioelectron. 2018, 102, 189–195. [Google Scholar] [CrossRef]
- Pei, F.; Wang, P.; Ma, E.; Yu, H.; Gao, C.; Yin, H.; Li, Y. A sandwich-type amperometric immunosensor fabricated by Au@Pd NDs/Fe2+-CS/PPy NTs and Au NPs/NH2-GS to detect CEA sensitively via two detection methods. Biosens. Bioelectron. 2018, 122, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, P.; Li, F.; Chu, Q.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of mesoporous core–shell Pd@Pt crossmark nanoparticles/amino group functionalized graphene nanocomposite. Biosens. Bioelectron. 2017, 87, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, P.; Pei, F.; Yu, H.; Dong, Y.; Li, Y.; Liu, Q.; Chen, P. A novel signal amplification system fabricated immunosensor based on Au nanoparticles and mesoporous trimetallic PdPtCu nanospheres for sensitive detection of prostate specific antigen. Sens. Actuators B Chem. 2018, 261, 22–30. [Google Scholar] [CrossRef]
- Miao, L.; Jiao, L.; Zhang, J.; Li, H. Amperometric sandwich immunoassay for the carcinoembryonic antigen using a glassy carbon electrode modified with iridium nanoparticles, polydopamine and reduced graphene oxide. Microchim. Acta 2017, 184, 169–175. [Google Scholar] [CrossRef]
- Hara, M.; Mallouk, T.E. Photocatalytic water oxidation by Nafion-stabilized iridium oxide colloids. Chem. Commun. 2000, 1903–1904. [Google Scholar] [CrossRef]
- Rivas, L.; de la Escosura-Muñiz, A.; Pons, J.; Merkoçi, A. Alzheimer Disease Biomarker Detection Through Electrocatalytic Water Oxidation Induced by Iridium Oxide Nanoparticles. Electroanalysis 2014, 26, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Quesada-González, D.; Baiocco, A.; Martos, A.A.; de la Escosura-Muñiz, A.; Merkoçi, A. Iridium oxide (IV) nanoparticle-based electrocatalytic detection of PBDE. Biosens. Bioelectron. 2019, 127, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.-C.; Gao, F.; Cui, Y.; Wang, W.; Luo, X. High-activity Fe3O4 nanozyme as signal amplifier: A simple, low-cost but efficient strategy for ultrasensitive photoelectrochemical immunoassay. Biosens. Bioelectron. 2019, 127, 64–71. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Bouša, D.; Mayorga-Martinez, C.C.; Mazánek, V.; Sofer, Z.; Boušova, K.; Pumera, M. MoS2 Nanoparticles as Electrocatalytic Labels in Magneto-Immunoassays. ACS Appl. Mater. Interfaces 2018, 10, 16861–16866. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Merkoçi, A. Nanochannels preparation and application in biosensing. ACS Nano 2012, 6, 7556–7583. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Merkoçi, A. Nanochannels for electrical biosensing. Trends Anal. Chem. 2016, 79, 134–150. [Google Scholar] [CrossRef] [Green Version]
- De la Escosura-Muñiz, A.; Merkoçi, A. A nanochannel/nanoparticle-based filtering and sensing platform for direct detection of a cancer biomarker in blood. Small 2011, 7, 675–682. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Merkoçi, A. Label-free voltammetric immunosensor using a nanoporous membrane based platform. Electrochem. Commun. 2010, 12, 859–863. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Espinoza-Castañeda, M.; Hasegawa, M.; Philippe, L.; Merkoçi, A. Nanoparticles-based nanochannels assembled on a plastic flexible substrate for label-free immunosensing. Nano Res. 2015, 8, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Castañeda, M.; de la Escosura-Muñiz, A.; Chamorro, A.; de Torres, C.; Merkoçi, A. Nanochannel array device operating through Prussian blue nanoparticles for sensitive label-free immunodetection of a cancer biomarker. Biosens. Bioelectron. 2015, 67, 107–114. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Merkoçi, A. Nanoparticle based enhancement of electrochemical DNA hybridization signal using nanoporous electrodes. Chem. Commun. 2010, 46, 9007–9009. [Google Scholar] [CrossRef] [Green Version]
- De la Escosura-Muñiz, A.; Chunglok, W.; Surareungchai, W.; Merkoçi, A. Nanochannels for diagnostic of thrombin-related diseases in human blood. Biosens. Bioelectron. 2013, 40, 24–31. [Google Scholar] [CrossRef] [Green Version]
- De la Escosura-Muñiz, A.; Espinoza-Castañeda, M.; Chamorro-García, A.; Rodríguez-Hernández, C.J.; de Torres, C.; Merkoçi, A. In situ monitoring of PTHLH secretion in neuroblastoma cells cultured onto nanoporous membranes. Biosens. Bioelectron. 2018, 107, 62–68. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Ivanova, K.; Tzanov, T. Electrical Evaluation of Bacterial Virulence Factors Using Nanopores. ACS Appl. Mater. Interfaces 2019, 11, 13140–13146. [Google Scholar] [CrossRef] [PubMed]












| NPs | Strategy | Analyte | LOD | Reference |
|---|---|---|---|---|
| AuNPs | Direct detection | Salmonella typhimurium | 7.7 cells/mL | [19] |
| AuNPs | Direct detection | CEA AFP | 4.6 pg/mL 3.1 pg/mL | [20] |
| AgNPs | Direct detection | Clenbuterol | 3.14 pg/mL | [26] |
| AgNPs | Direct detection | TBEV | 50 IU/mL | [27] |
| AuAgNPs | Direct detection | E. coli | 102 CFU/mL | [30] |
| CdSe@ZnS QDs | Direct detection | anti-tTG IgG | 2.2 U/mL | [41] |
| CdSe@ZnS QDs | Direct detection | anti-tTG IgA | 2.4 U/mL | [42] |
| CdSe@ZnS QDs | Direct detection | ApoE | 12.5 ng/mL | [45] |
| CdSe@ZnS QDs | Direct detection | HIgG | 3.5 ng/mL | [46] |
| SA/Au/CNH | Silver electrodeposition-based detection | CEA AFP | 32 fg/mL 24 fg/mL | [76] |
| AuNPs | HER-based detection | ApoE Beta amyloid | 80 pg/mL 19 pg/mL | [83] |
| AuNPs | HER-based detection | E. coli O157:H7 | 457 CFU/mL (in minced beef) 309 CFU/mL (in tap water) | [84] |
| Na-Mont-PANI-AuNPs | H2O2 reduction-based detection | SCC-Ag | 300 fg/mL | [87] |
| PS@PEI@CAT@AuNPs | H2O2 reduction-based detection | TBBPA-DHEE | 120 pg/mL | [88] |
| AgNPs@CS-Hemin/rGO | H2O2 reduction-based detection | CEA | 6.7 fg/mL | [89] |
| MoS2@Cu2O-PtNPs | H2O2 reduction-based detection | HBsAg | 150 fg/mL | [90] |
| PtCu@rGO/g-C3N4 | H2O2 reduction-based detection | PSA | 16.6 fg/mL | [91] |
| Ag@Au-Fe3O4 | H2O2 reduction-based detection | HIgG | 50 fg/mL | [92] |
| Rh@Pt NDs/NH2-GS | H2O2 reduction-based detection | HBsAg | 166 fg/mL | [95] |
| PtNPs/Co3O4/graphene | H2O2 reduction-based detection | AFP | 29 fg/mL | [96] |
| Pd/APTES-M-CeO2-GS | H2O2 reduction-based detection | AFP | 33 fg/mL | [97] |
| Pd/NH2-ZIF-67 | H2O2 reduction-based detection | PSA | 30 fg/mL | [98] |
| g-C3N4-PdNPs | H2O2 reduction-based detection | STX | 1.2 pg/mL | [100] |
| Au@Ag/Au core@double shell NPs | H2O2 reduction-based detection | SCC-Ag | 180 fg/mL | [104] |
| Co3O4@CeO2-Au@Pt | H2O2 reduction-based detection | SCC-Ag | 33 fg/mL | [105] |
| Au@Ag-Cu2O | H2O2 reduction-based detection | PSA | 3 fg/mL | [106] |
| Au@Ag/PDA-PR-MCS | H2O2 reduction-based detection | AFP | 6.7 fg/mL | [107] |
| rGO-NR Au@Pt | H2O2 reduction-based detection | E. coli O157:H7 | 91 CFU/mL | [108] |
| Au@Pt DNs/NG/ Cu2+ | H2O2 reduction-based detection | CEA | 167 fg/mL | [109] |
| Au@Pd/MoS2@MWCNTs | H2O2 reduction-based detection | HBeAg | 26 fg/mL | [110] |
| Au@Pd NDs/Fe2+-CS/PPy NTs | Direct detection H2O2 reduction-based detection | CEA | 167 fg/mL 17 fg/mL | [111] |
| M-Pd@Pt/NH2-GS | H2O2 reduction-based detection | PSA | 3.3 fg/mL | [112] |
| m-PdPtCu | H2O2 reduction-based detection | PSA | 3.3 fg/mL | [113] |
| IrNPs | H2O2 reduction-based detection | CEA | 230 fg/mL | [114] |
| IrO2 NPs | Water oxidation reaction-based detection | ApoE | 68 ng/mL | [116] |
| IrO2 NPs | Water oxidation reaction-based detection | PBDE | 21.5 ppb | [117] |
| his-Fe3O4 | H2O2 reduction-based detection | PSA | 18 fg/mL | [120] |
| MoS2 NPs | Hydrogen evolution reaction-based detection | Rabbit IgG | 1.94 pg/mL | [122] |
| AuNPs | Nanochannel blocking agent | HIgG | 580 ng/mL | [127] |
| AuNPs | Nanochannel blocking agent | PTHrP | 50 ng/mL | [128] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as Emerging Labels in Electrochemical Immunosensors. Sensors 2019, 19, 5137. https://0-doi-org.brum.beds.ac.uk/10.3390/s19235137
Iglesias-Mayor A, Amor-Gutiérrez O, Costa-García A, de la Escosura-Muñiz A. Nanoparticles as Emerging Labels in Electrochemical Immunosensors. Sensors. 2019; 19(23):5137. https://0-doi-org.brum.beds.ac.uk/10.3390/s19235137
Chicago/Turabian StyleIglesias-Mayor, Alba, Olaya Amor-Gutiérrez, Agustín Costa-García, and Alfredo de la Escosura-Muñiz. 2019. "Nanoparticles as Emerging Labels in Electrochemical Immunosensors" Sensors 19, no. 23: 5137. https://0-doi-org.brum.beds.ac.uk/10.3390/s19235137