A Comparative Assessment of Measures of Leaf Nitrogen in Rice Using Two Leaf-Clip Meters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Agronomical Data Acquisition and Determination
2.2.1. Sampling Period and N Concentration Determination
2.2.2. SPAD and Dualex Values Measurements
2.2.3. Chlorophyll (Chl) and Flavonoid (Flav) Content Determination
2.2.4. Nitrogen Nutrition Index (NNI)
2.2.5. Nitrogen Sufficiency Index (NSI)
2.2.6. Accumulated Nitrogen Deficit (AND)
2.2.7. Calculation of Relative Accumulated Growing Degree Days (RAGDD)
2.2.8. Statistical Analysis
3. Results
3.1. Relationship between Leaf-Clip Values (Flav, Chl, and SPAD) and Measured Values in Leaves
3.2. Sensitivity Analysis of Dualex Meter and SPAD Meter in N Nutrition Status
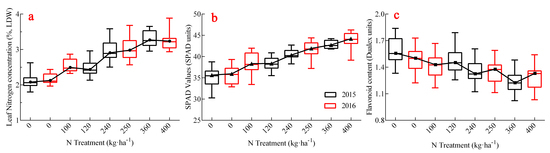
3.3. Dynamic Characteristics of Dualex and SPAD Values Under Different Treatments
3.4. Relationship between N Indicators and Meters’ Index (Dualex Meter: Flav, Chl, and NBI; SPAD Meter: SPAD Value)
3.5. The Threshold of Conventional Japonica Rice N Application Based on Flav Value
4. Discussion
4.1. Chlorophyll Estimation Based on Dualex and SPAD
4.2. Discrimination and Sensitivity of Rice Nitrogen Levels by Dualex Meter and SPAD Meter
4.3. The Threshold of Rice Nitrogen Nutrition Diagnostics Based on Dualex
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RMSE | root mean square error |
| RRMSE | relative RMSE |
| AND | accumulated nitrogen deficit |
| NBI | nitrogen balance index |
| Flav | flavonoid |
| Chl | chlorophyll |
| TI | tillering |
| SE | stem elongation |
| PI | panicle initiation |
| BT | booting |
| HD | heading |
| GF | grain filling |
| NNI | nitrogen nutrition index |
| NSI | nitrogen sufficiency index |
| RAGDD | calculation of Relative Accumulated Growing Degree Days |
References
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO 2002, 31, 132. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 292. [Google Scholar] [CrossRef]
- Lundström, C.; Lindblom, J.; Hansen, J.W.; Thornton, P.K.; Berentsen, P.B.M. Considering farmers’ situated knowledge of using agricultural decision support systems (AgriDSS) to Foster farming practices: The case of CropSAT. Agric. Syst. 2018, 159, 9–20. [Google Scholar] [CrossRef]
- Li, F.; Etc, Y.M. Estimating winter wheat biomass and nitrogen status using an active crop sensor. Intell. Autom. Soft Comput. 2010, 16, 1221–1230. [Google Scholar]
- Clay, D.E.; Kharel, T.P.; Reese, C.; Beck, D.; Carlson, C.G.; Clay, S.A.; Reicks, G. Winter Wheat Crop Reflectance and Nitrogen Sufficiency Index Values are Influenced by Nitrogen and Water Stress. Agron. J. 2012, 104, 1612. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Perry, E.M.; Davenport, J.R. Spectral and spatial differences in response of vegetation indices to nitrogen treatments on apple. Comput. Electron. Agric. 2007, 59, 56–65. [Google Scholar] [CrossRef]
- Foster, A.J.; Kakani, V.G.; Ge, J.; Mosali, J. Discrimination of switchgrass cultivars and nitrogen treatments using pigment profiles and hyperspectral leaf reflectance data. Remote Sens. 2012, 4, 2576–2594. [Google Scholar] [CrossRef] [Green Version]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Yao, X.; Deng, X.; Tian, Y.; Cao, W.; Zhu, Y. Detection of rice phenology through time series analysis of ground-based spectral index data. Field Crop. Res. 2016, 198, 131–139. [Google Scholar] [CrossRef]
- Wang, S.H.; Cao, W.X.; Wang, Q.S.; Ding, Y.F.; Huang, P.S.; Ling, Q.H. Positional distribution of leaf color and diagnosis of nitrogen nutrition in rice plant. Sci. Agric. Sin. 2002, 192, 45–51. [Google Scholar]
- Wang, S.; Zhu, Y.; Jiang, H.; Cao, W. Positional differences in nitrogen and sugar concentrations of upper leaves relate to plant N status in rice under different N rates. Field Crop. Res. 2006, 96, 224–234. [Google Scholar] [CrossRef]
- Lin, F.F.; Qiu, L.F.; Deng, J.S.; Shi, Y.Y.; Chen, L.S.; Ke, W. Investigation of SPAD meter-based indices for estimating rice nitrogen status. Comput. Electron. Agric. 2010, 71, S60–S65. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Wang, K.; Zhu, J.Y. Preliminary study on diagnosis of the nitrogen status of two rice varieties using the chlorophyll meter. Bull. Sci. Technol. 2002, 18, 174–176. [Google Scholar]
- Yuan, Z.; Ata-Ul-Karim, S.T.; Cao, Q.; Lu, Z.; Cao, W.; Zhu, Y.; Liu, X. Indicators for diagnosing nitrogen status of rice based on chlorophyll meter readings. Field Crop. Res. 2016, 185, 12–20. [Google Scholar] [CrossRef]
- Wu, J.D.; Wang, D.; Rosen, C.J.; Bauer, M.E. Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crop. Res. 2007, 101, 96–103. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Cai, H.; Zhou, W.; Xu, F. H2O2 mediates nitrate-induced iron chlorosis by regulating iron homeostasis in rice. Plant Cell Environ. 2018, 41, 767–781. [Google Scholar] [CrossRef]
- Schijlen, E.G.W.M.; Vos, C.H.R.D.; Tunen, A.J.V.; Bovy, A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry 2004, 65, 2631–2648. [Google Scholar] [CrossRef]
- Cui, X.H.; Paek, K.Y. Adventitious root suspension cultures of Hypericum perforatum: Effect of nitrogen source on production of biomass and secondary metabolites. Vitro Cell. Dev. Biol.-Plant 2010, 46, 437–444. [Google Scholar] [CrossRef]
- Strissel, T.; Halbwirth, H.; Hoyer, U.; Zistler, C.; Stich, K.; Treutter, D. Growth-promoting nitrogen nutrition affects flavonoid biosynthesis in young apple (Malus domestica Borkh.) leaves. Plant Biol. 2005, 7, 677. [Google Scholar] [CrossRef]
- Winkelshirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Bilo 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Dong, X.; Chen, W.; Wang, W.; Zhang, H.; Liu, X.; Luo, J. Comprehensive profiling and natural variation of flavonoids in rice. J. Integr. Plant Biol. 2014, 56, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Zebarth, B.J.; Drury, C.F.; Tremblay, N.; Cambouris, A.N. Opportunities for improved fertilizer nitrogen management in production of arable crops in eastern Canada: A review. Can. J. Soil Sci. 2010, 89, 113–132. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, J.; Zhao, B.Q.; Zhang, D.Y.; Xie, J. Research on the Chlorophyll Content (SPAD) Distribution Based on the Consumer-Grade Modified Near-Infrared Camera. Spectrosc. Spectr. Anal. 2018, 38, 737–744. [Google Scholar]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, H.M.; Gate, P.; Agati, G. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Zhu, J.; Tremblay, N.; Liang, Y. A corn nitrogen status indicator less affected by soil water content. Agron. J. 2011, 103, 890. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Quemada, M.; Alonso-Ayuso, M.; Lizaso, J.I.; Martín-Lammerding, D. Predicting N Status in Maize with Clip Sensors: Choosing Sensor, Leaf Sampling Point, and Timing. Sensors 2019, 19, 3881. [Google Scholar] [CrossRef] [Green Version]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal Optical Sensors for Nitrogen Management of Vegetable Crops: A Review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ge, X.; Liu, X.; Zhang, Z.; Liang, Y.; Tian, Y.; Cao, Q.; Cao, W.; Zhu, Y.; Liu, X. Evaluation of the Chlorophyll Meter and GreenSeeker for the Assessment of Rice Nitrogen Status. Adv. Anim. Biosci. 2017, 8, 359–363. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, X.; Shen, P.; Li, W.; Tian, Y. Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages. Remote Sens. 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Ataulkarim, S.T.; Liu, X.; Lu, Z.; Yuan, Z.; Yan, Z.; Cao, W. In-season estimation of rice grain yield using critical nitrogen dilution curve. Field Crop. Res. 2016, 195, 1–8. [Google Scholar]
- Yuan, Z.; Qiang, C.; Ke, Z.; Ata-Ul-Karim, S.T.; Tian, Y.; Yan, Z.; Cao, W.; Liu, X. Optimal leaf positions for spad meter measurement in rice. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wei, Y.S.; Wang, Y.N.; Shi, Y.P.; Ting, L.U. A study on experimental conditions in determining total flavonoids by spectrophotometry. J. Qinghai Univ. 2003, 21, 61–63. [Google Scholar]
- Ata-Ul-Karim, S.T.; Xia, Y.; Liu, X.; Cao, W.; Yan, Z. Development of critical nitrogen dilution curve of Japonica rice in Yangtze River Reaches. Field Crop. Res. 2013, 149, 149–158. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Liu, X.; Lu, Z.; Zheng, H.; Cao, W.; Zhu, Y. Estimation of nitrogen fertilizer requirement for rice crop using critical nitrogen dilution curve. Field Crop. Res. 2017, 201, 32–40. [Google Scholar] [CrossRef]
- Russelle, M.P.; Wilhelm, W.W.; Olson, R.A.; Power, J.F. Growth analysis based on degree days. Crop Sci. 1984, 24, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Miao, Y.; Huang, S.; Gao, L.; Ma, X.; Zhao, G.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Thind, H.S.; Kumar, A.; Gupta, R.K.; Kaul, A.; Vashistha, M. Fixed-time adjustable dose site-specific fertilizer nitrogen management in transplanted irrigated rice (Oryza sativa L.) in South Asia. Field Crop. Res. 2012, 126, 63–69. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q. Improving Nitrogen Fertilization in Rice by Site-Specific MN Management. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, É.; Renaud, A.; Richardson, A.D.; Roggy, J.C.; Schimann, H.; Uddling, J.; Hérault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef] [Green Version]
- Pilonsmits, E. Annual review of plant biology. Annu. Rev. Plant Biol. 2005, 56, 331–334. [Google Scholar]
- Carter, G.A.; Spiering, B.A. Optical properties of intact leaves for estimating chlorophyll concentration. J. Environ. Qual. 2002, 31, 1424–1432. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Yang, J.; Zou, Y.; Zhong, X.; Wang, G.; Zhang, F. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop. Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving in-season estimation of rice yield potential and responsiveness to topdressing nitrogen application with Crop Circle active crop canopy sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- René, G.; Birte, B. Validity of accessible critical nitrogen dilution curves in perennial ryegrass for seed production. Field Crop. Res. 2009, 111, 152–156. [Google Scholar]
- Ata-Ul-Karim, S.T.; Zhu, Y.; Liu, X.; Cao, Q.; Tian, Y.; Cao, W. Comparison of different critical nitrogen dilution curves for nitrogen diagnosis in rice. Sci. Rep. 2017, 7, 42679. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Garcia, F.V.; Laza, R.C.; Sanico, A.L.; Visperas, R.M.; Cassman, K.G. Increased N-use efficiency using a chlorophyll meter on high-yielding irrigated rice. Field Crop. Res. 1996, 47, 243–252. [Google Scholar] [CrossRef]









| Number of Experiment | Location | Transplanting/Harvest Date | Cultivar | Treatment (kg·hm−2) | Sampling Date | Soil Classification |
|---|---|---|---|---|---|---|
| Day/Month | ||||||
| Experiment.1 2015 | SiHong 118.26° E, 33.37° N | 14 June | WYJ-24, NJ-4, LJ-7 | N0 = 0 | 23 July, 30 July | Lime concretion blacks soil |
| 25 October | N2 = 120 | 6 August, 13 August | Total N = 1.28 g kg−1 | |||
| N3 = 240 | 19 August, 24 August | Olsen P = 27.6 mg kg−1 | ||||
| N5 = 360 | 12 September | Available K = 75.2 mg kg−1 | ||||
| Experiment.2 2015 | Huai’An 118.89° E, 33.59° N | 22 June | WYJ-24, NJ-4, LJ-7 | N0 = 0 | 25 July, 1 August | Yellow-brown soil |
| 1 November | N2 = 120 | 8 August, 15 August | Total N = 1.35 g kg−1 | |||
| N3 = 240 | 21 August, 28 August | Olsen P = 32 mg kg−1 | ||||
| N5 = 360 | 10 September | Available K = 85.3 mg kg−1 | ||||
| Experiment.3 2016 | SiHong 118.26° E, 33.37° N | 25 June | WYJ-24, NJ-4, LJ-7 | N0 = 0 | 22 July, 4 August | Lime concretion blacks soil |
| 26 October | N2 = 120 | 15 August, 22 August | Total N = 1.28 g kg−1 | |||
| N3 = 240 | 28 August, 10 September | Olsen P = 27.6 mg kg−1 | ||||
| N5 = 360 | Available K = 75.2 mg kg−1 | |||||
| Experiment.4 2016 | RuGao 120.76° E, 32.27° N | 18 June | WYJ-24, NJ-4 | N0 = 0 | 16 July, 25 July | Loam soil |
| 22 October | N1 = 100 | 2 August, 11 August | Total N = 1.66 g kg−1 | |||
| N4 = 250 | 21 August, 26 August | Olsen P = 13.6 mg kg−1 | ||||
| N6 = 400 | 21 September | Available K = 92.6 mg kg−1 |
| N Indicator | Meters’ Indicator | Growth Stage | ||||||
|---|---|---|---|---|---|---|---|---|
| Tillering | Stem Elongation | Panicle Initiation | Booting | Heading | Flowering | Total | ||
| LNC | FLAV | 0.52 ** | 0.77 ** | 0.83 ** | 0.75 ** | 0.71 ** | 0.62 ** | 0.79 ** |
| Chl | 0.49 ** | 0.67 ** | 0.80 ** | 0.72 ** | 0.64 ** | 0.49 * | 0.73 ** | |
| NBI | 0.47 ** | 0.69 ** | 0.72 ** | 0.67 ** | 0.67 ** | 0.45 * | 0.75 ** | |
| SPAD | 0.35 ** | 0.76 ** | 0.74 ** | 0.63 * | 0.73 ** | 0.61 ** | 0.70 ** | |
| PNC | FLAV | 0.59 ** | 0.74 ** | 0.76 ** | 0.74 ** | 0.70 ** | 0.56 ** | 0.69 ** |
| Chl | 0.53 ** | 0.61 ** | 0.74 ** | 0.71 ** | 0.70 ** | 0.43 * | 0.52 ** | |
| NBI | 0.55 ** | 0.62 ** | 0.73 ** | 0.77 ** | 0.72 ** | 0.54 ** | 0.68 ** | |
| SPAD | 0.51 ** | 0.73 ** | 0.72 ** | 0.69 ** | 0.63 ** | 0.48 * | 0.60 ** | |
| NNI | FLAV | 0.68 ** | 0.73 ** | 0.79 ** | 0.82 ** | 0.79 ** | 0.72 ** | 0.73 ** |
| Chl | 0.66 ** | 0.68 ** | 0.79 ** | 0.76 ** | 0.68 ** | 0.72 ** | 0.66 ** | |
| NBI | 0.63 ** | 0.58 ** | 0.84 ** | 0.82 ** | 0.73 ** | 0.56 ** | 0.69 ** | |
| SPAD | 0.58 ** | 0.62 ** | 0.79 ** | 0.72 ** | 0.71 ** | 0.58 ** | 0.67 ** | |
| NSI | FLAV | 0.53 ** | 0.76 ** | 0.84 ** | 0.78 ** | 0.72 ** | 0.66 ** | 0.78 ** |
| Chl | 0.60 ** | 0.62 ** | 0.76 ** | 0.68 ** | 0.65 ** | 0.34 * | 0.70 ** | |
| NBI | 0.57 * | 0.61 ** | 0.67 ** | 0.84 ** | 0.64 ** | 0.42 * | 0.74 ** | |
| SPAD | 0.45 ** | 0.66 ** | 0.76 ** | 0.67 * | 0.70 ** | 0.64 ** | 0.65 ** | |
| AND | FLAV | 0.72 ** | 0.73 ** | 0.73 ** | 0.71 ** | 0.66 ** | 0.72 ** | 0.71 ** |
| Chl | 0.65 ** | 0.64 ** | 0.55 ** | 0.61 ** | 0.61 ** | 0.66 * | 0.63 ** | |
| NBI | 0.68 ** | 0.71 ** | 0.63 ** | 0.62 ** | 0.58 ** | 0.72 * | 0.69 ** | |
| SPAD | 0.55 ** | 0.64 ** | 0.65 ** | 0.68 * | 0.62 ** | 0.68 ** | 0.65 ** | |
| Grain yield | FLAV | 0.58 ** | 0.53 ** | 0.56 ** | 0.48 * | 0.64 ** | 0.63 ** | 0.49 * |
| Chl | 0.56 ** | 0.52 ** | 0.53 ** | 0.12 | 0.52 ** | 0.62 ** | 0.41 * | |
| NBI | 0.58 ** | 0.50 ** | 0.48 * | 0.30 * | 0.49 * | 0.61 ** | 0.46 * | |
| SPAD | 0.49 ** | 0.61 ** | 0.44 * | 0.49 * | 0.53 ** | 0.64 ** | 0.47 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Liu, X.; Ma, Y.; Zhang, R.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. A Comparative Assessment of Measures of Leaf Nitrogen in Rice Using Two Leaf-Clip Meters. Sensors 2020, 20, 175. https://0-doi-org.brum.beds.ac.uk/10.3390/s20010175
Zhang K, Liu X, Ma Y, Zhang R, Cao Q, Zhu Y, Cao W, Tian Y. A Comparative Assessment of Measures of Leaf Nitrogen in Rice Using Two Leaf-Clip Meters. Sensors. 2020; 20(1):175. https://0-doi-org.brum.beds.ac.uk/10.3390/s20010175
Chicago/Turabian StyleZhang, Ke, Xiaojun Liu, Yong Ma, Rui Zhang, Qiang Cao, Yan Zhu, Weixing Cao, and Yongchao Tian. 2020. "A Comparative Assessment of Measures of Leaf Nitrogen in Rice Using Two Leaf-Clip Meters" Sensors 20, no. 1: 175. https://0-doi-org.brum.beds.ac.uk/10.3390/s20010175