Entropy of Real-World Gait in Parkinson’s Disease Determined from Wearable Sensors as a Digital Marker of Altered Ambulatory Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics and Consent
2.3. Demographic and Clinical Measures
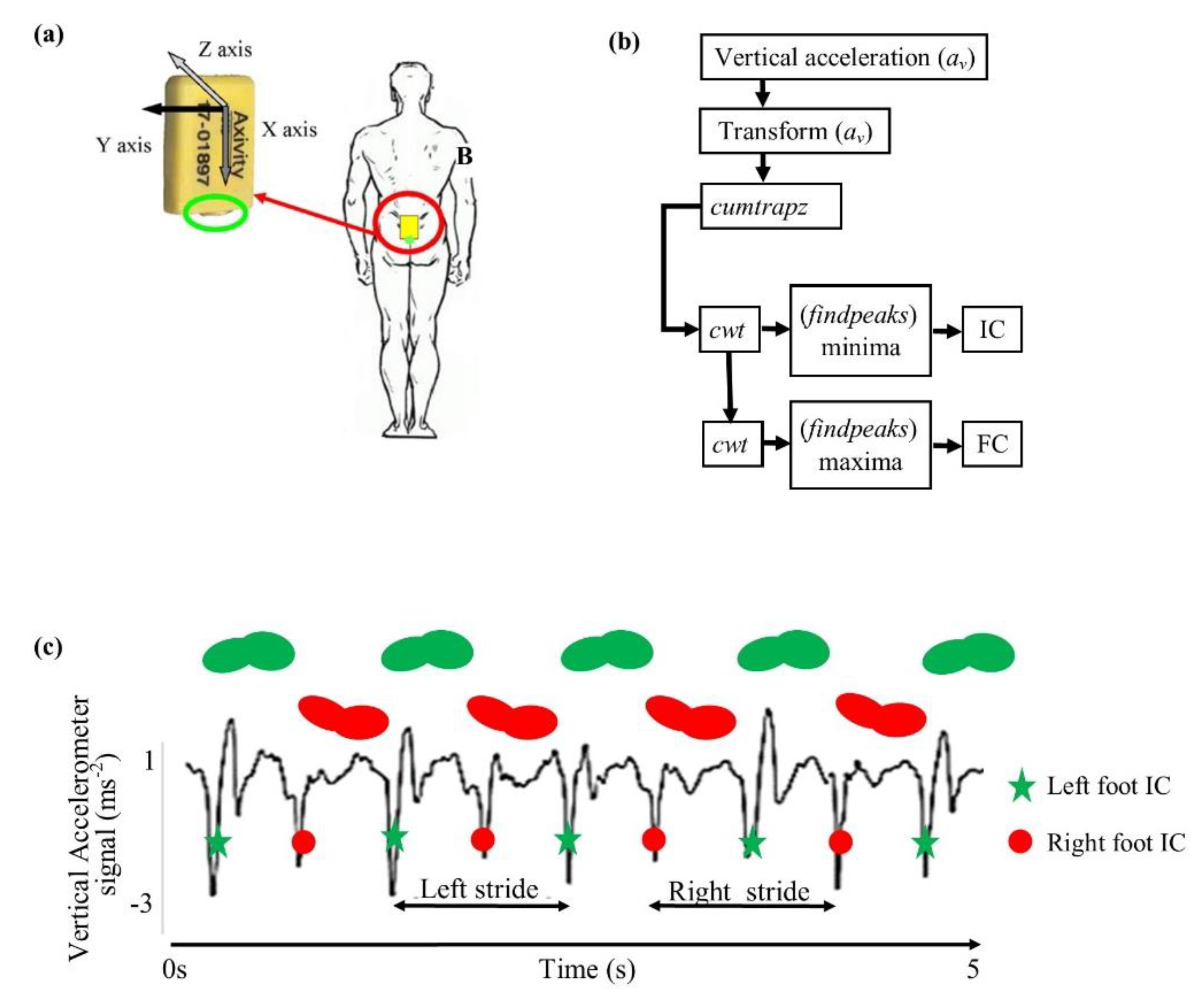
2.4. Real-World Data: Equipment and Procedure
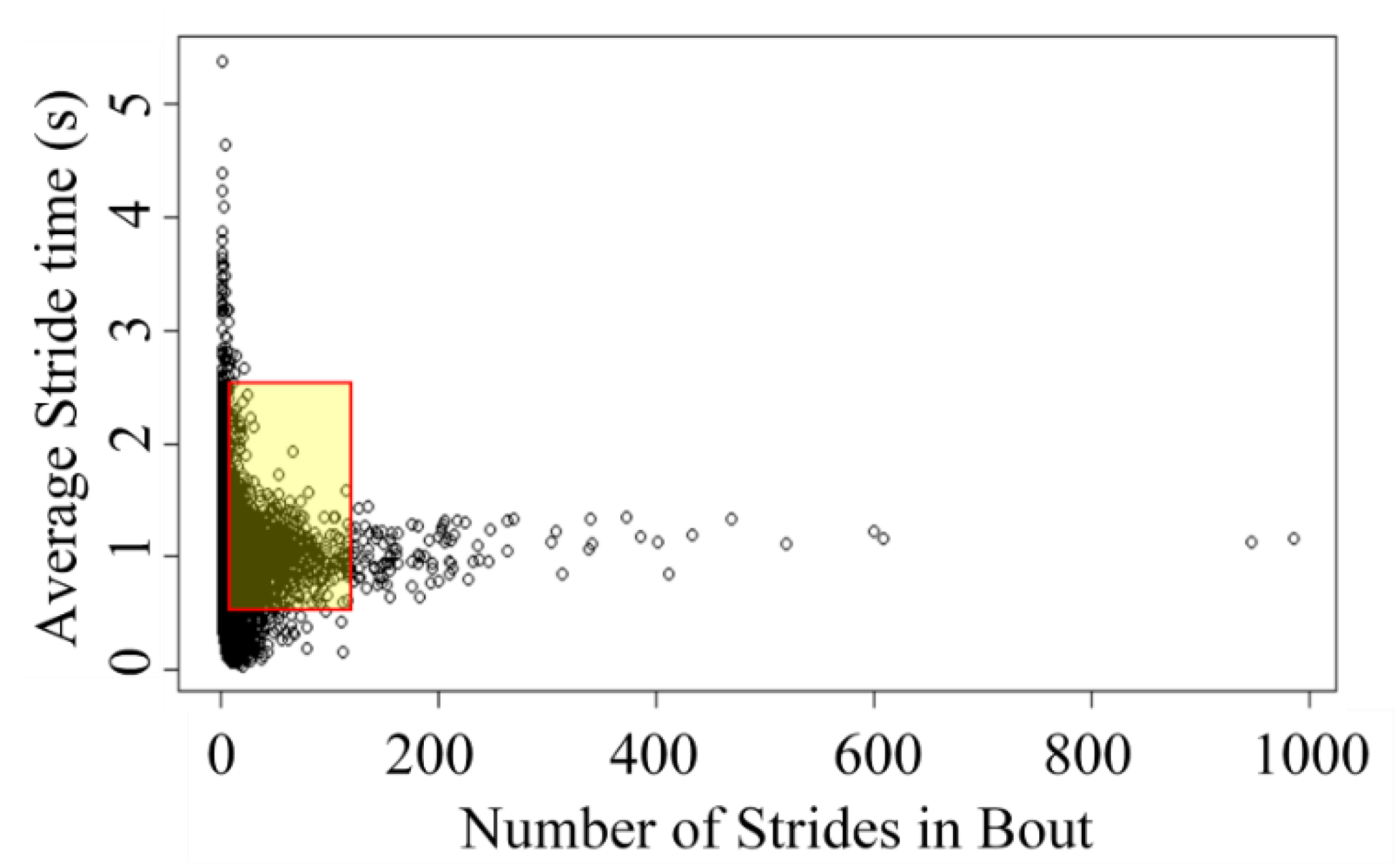
2.5. Data Processing and Analysis
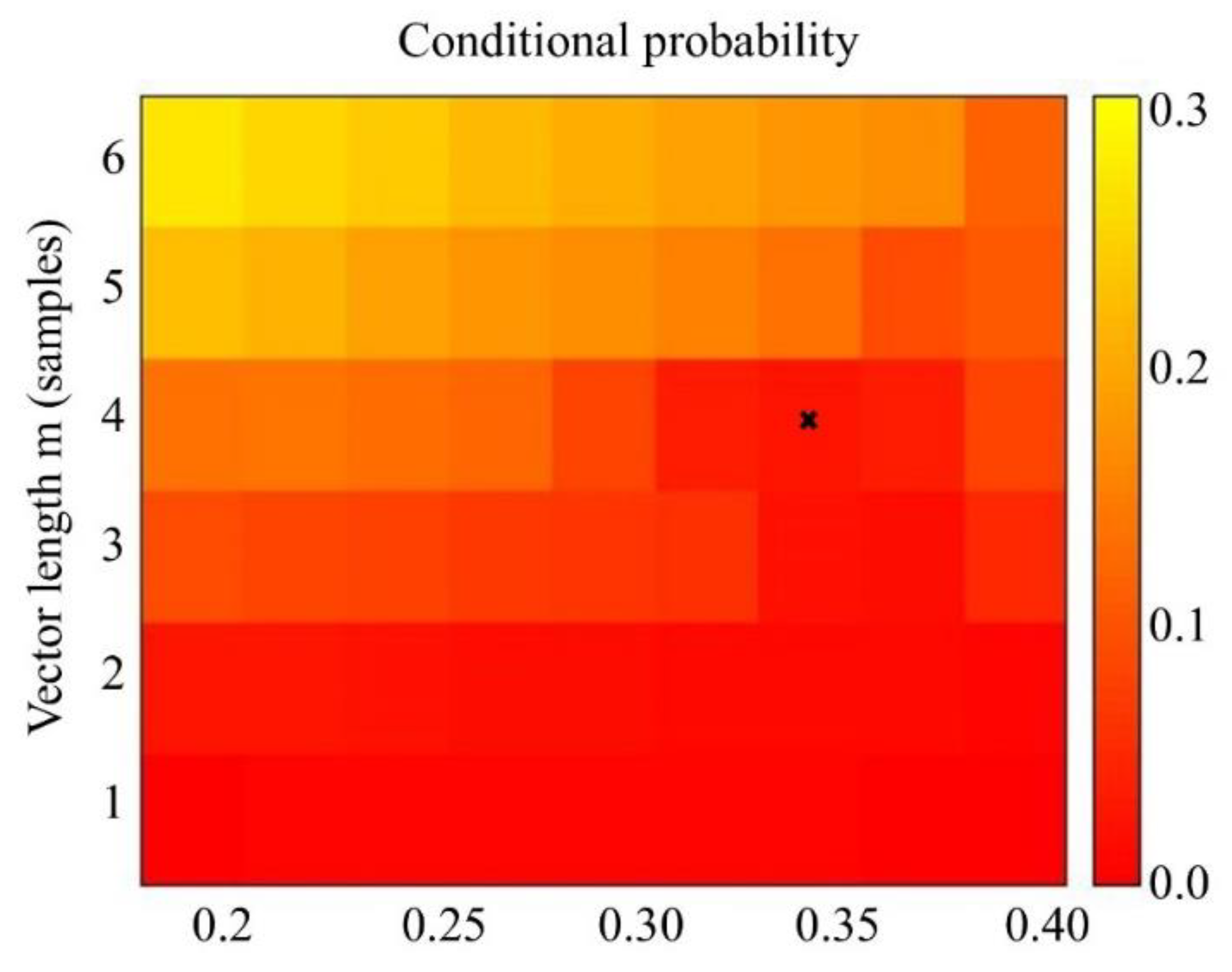
2.6. Calculation of Sample Entropy
2.7. Determining Nonlinearity
2.8. Statistical Analysis
3. Results
3.1. Demographics
3.2. Surrogate Analysis
3.3. Sample Entropy
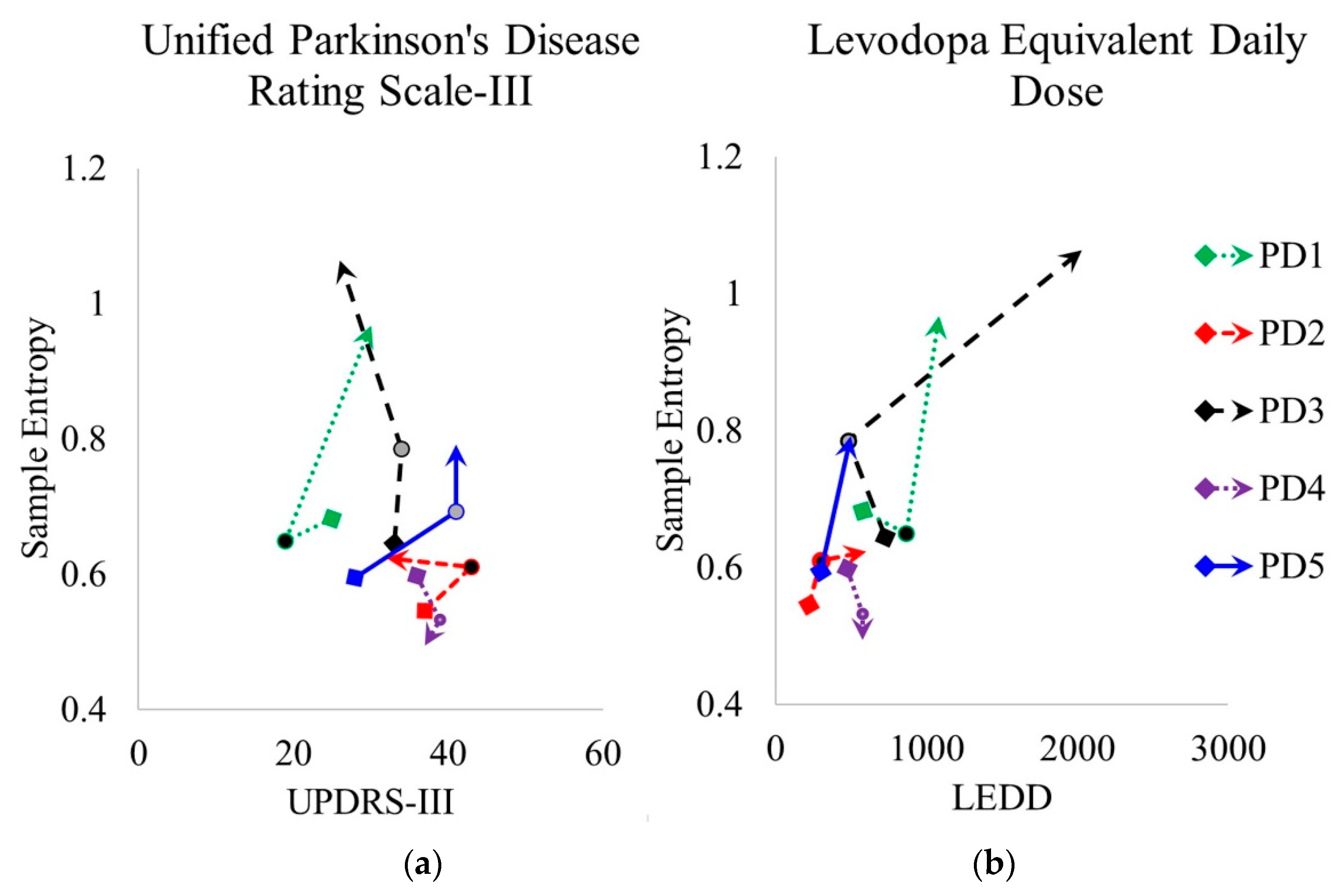
3.4. Clinical Features and Sample Entropy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BWM | Body worn monitor |
| CV | Coefficient of variation |
| HOA | Healthy older adults |
| LRA | Long range autocorrelation |
| MDS-UPDRS | Movement Disorder Society-Unified Parkinson’s Disease Rating Scale |
| PD | Parkinson’s disease |
| SampEnt | Sample entropy |
| TP1 | Time-point 1 (36 months after initial diagnosis for people with Parkinson’s) |
| TP2 | Time-point 2 = TP1 + 18 months |
| TP3 | Time-point 2 = TP2 + 18 months |
References
- Lunenfeld, B.; Stratton, P. The clinical consequences of an ageing world and preventive strategies. Best Pr. Res. Clin. Obs. Gynaecol. 2013, 27, 643–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsfall, L.; Petersen, I.; Walters, K.; Schrag, A. Time trends in incidence of Parkinson’s disease diagnosis in UK primary care. J. Neurol. 2013, 260, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Yarnall, A.J.; Morris, R.; Coleman, S.; Burn, D.; Rochester, L. Natural history of falls in an incident cohort of Parkinson’s disease: Early evolution, risk and protective features. J. Neurol. 2017, 264, 2268–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Din, S.; Godfrey, A.; Galna, B.; Lord, S.; Rochester, L. Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J. Neuroeng. Rehabil. 2016, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Robles-Garcia, V.; Corral-Bergantinos, Y.; Espinosa, N.; Jacome, M.A.; Garcia-Sancho, C.; Cudeiro, J.; Arias, P. Spatiotemporal Gait Patterns During Overt and Covert Evaluation in Patients With Parkinson s Disease and Healthy Subjects: Is There a Hawthorne Effect? J. Appl. Biomech. 2015, 31, 189–194. [Google Scholar] [CrossRef]
- Del Din, S.; Hickey, A.; Hurwitz, N.; Mathers, J.C.; Rochester, L.; Godfrey, A. Measuring gait with an accelerometer-based wearable: Influence of device location, testing protocol and age. Physiol. Meas. 2016, 37, 1785–1797. [Google Scholar] [CrossRef]
- Del Din, S.; Hickey, A.; Woodman, S.; Hiden, H.; Morris, R.; Watson, P.; Nazarpour, K.; Catt, M.; Rochester, L.; Godfrey, A. Accelerometer based gait assessment: Pragmatic deployment on an international scale. In Proceedings of the 2016 IEEE Statistical Signal Processing Workshop (SSP), Palma de Mallorca, Spain, 26–29 June 2016. [Google Scholar]
- Baker, K.; Rochester, L.; Nieuwboer, A. The effect of cues on gait variability—Reducing the attentional cost of walking in people with Parkinson’s disease. Parkinsonism Relat. Disord. 2008, 14, 314–320. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng. Rehabil. 2005, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Hausdorff, J.M.; Schaafsma, J.D.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Giladi, N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp. Brain Res. 2003, 149, 187–194. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J. Neurol. Sci. 2003, 212, 47–53. [Google Scholar] [CrossRef]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 2012, 59, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Warlop, T.; Detrembleur, C.; Buxes Lopez, M.; Stoquart, G.; Lejeune, T.; Jeanjean, A. Does Nordic Walking restore the temporal organization of gait variability in Parkinson’s disease? J. Neuroeng. Rehabil. 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 2009, 19, 026113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, C. A novel perspective to assessment of neurodegenerative disorder gait. Sci. Postprint 2015, 1, e00051. [Google Scholar] [CrossRef]
- Warlop, T.; Detrembleur, C.; Bollens, B.; Stoquart, G.; Crevecoeur, F.; Jeanjean, A.; Lejeune, T.M. Temporal organization of stride duration variability as a marker of gait instability in Parkinson’s disease. J. Rehabil. Med. 2016, 48, 865–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantall, A.; Del Din, S.; Rochester, L. Longitudinal changes over thirty-six months in postural control dynamics and cognitive function in people with Parkinson’s disease. Gait Posture 2018, 62, 468–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihlen, E.A.F.; van Schooten, K.S.; Bruijn, S.M.; van Dieen, J.H.; Vereijken, B.; Helbostad, J.L.; Pijnappels, M. Improved Prediction of Falls in Community-Dwelling Older Adults Through Phase-Dependent Entropy of Daily-Life Walking. Front. Aging Neurosci. 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Ihlen, E.A.F.; Weiss, A.; Bourke, A.; Helbostad, J.L.; Hausdorff, J.M. The complexity of daily life walking in older adult community-dwelling fallers and non-fallers. J. Biomech. 2016, 49, 1420–1428. [Google Scholar] [CrossRef]
- Alcaraz, R.; Abasolo, D.; Hornero, R.; Rieta, J.J. Optimal parameters study for sample entropy-based atrial fibrillation organization analysis. Comput. Methods Programs Biomed. 2010, 99, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Duan, F.; Yang, Y.; Sun, Z. The Effect of Treadmill Walking on Gait and Upper Trunk through Linear and Nonlinear Analysis Methods. Sensors 2019, 19, 2204. [Google Scholar] [CrossRef] [Green Version]
- Sokunbi, M.O. Sample entropy reveals high discriminative power between young and elderly adults in short fMRI data sets. Front. Neuroinform. 2014, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Coleman, S.; O’Brien, J.T.; Brooks, D.J.; Barker, R.A.; Burn, D.J. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013, 80, 276–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Ladha, C.; Ladha, K.; Jackson, D.; Olivier, P. Shaker table validation of OpenMovement AX3 accelerometer. In Proceedings of the 3rd International Conference on Ambulatory Monitoring of Physical Activity and Movement, Ahmerst, MA, USA, 17–19 June 2013; pp. 69–70. [Google Scholar]
- Hickey, A.; Del Din, S.; Rochester, L.; Godfrey, A. Detecting free-living steps and walking bouts: Validating an algorithm for macro gait analysis. Physiol. Meas. 2017, 38, N1–N15. [Google Scholar] [CrossRef]
- Moe-Nilssen, R. A new method for evaluating motor control in gait under real-life environmental conditions. Part 1: The instrument. Clin. Biomech. (Bristolavon) 1998, 13, 320–327. [Google Scholar] [CrossRef]
- Lyons, G.M.; Culhane, K.M.; Hilton, D.; Grace, P.A.; Lyons, D. A description of an accelerometer-based mobility monitoring technique. Med. Eng. Phys. 2005, 27, 497–504. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: Toward clinical and at home use. IEEE J. Biomed. Health Inform. 2016, 20, 838–847. [Google Scholar] [CrossRef]
- Godfrey, A.; Del Din, S.; Barry, G.; Mathers, J.C.; Rochester, L. Instrumenting gait with an accelerometer: A system and algorithm examination. Med. Eng. Phys. 2015, 37, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, A.; Lord, S.; Galna, B.; Mathers, J.C.; Burn, D.J.; Rochester, L. The association between retirement and age on physical activity in older adults. Age Ageing 2014, 43, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Najafi, B.; Aminian, K.; Paraschiv-Ionescu, A.; Loew, F.; Bula, C.J.; Robert, P. Ambulatory system for human motion analysis using a kinematic sensor: Monitoring of daily physical activity in the elderly. IEEE Trans. Biomed. Eng. 2003, 50, 711–723. [Google Scholar] [CrossRef]
- Richman, J.S.; Lake, D.E.; Moorman, J.R. Sample entropy. Methods Enzym. 2004, 384, 172–184. [Google Scholar]
- Yentes, J.M.; Denton, W.; McCamley, J.; Raffalt, P.C.; Schmid, K.K. Effect of parameter selection on entropy calculation for long walking trials. Gait Posture 2018, 60, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef] [Green Version]
- Lake, D.E.; Richman, J.S.; Griffin, M.P.; Moorman, J.R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef] [Green Version]
- Stergiou, N. Nonlinear Analysis for Human Movement Variability; CRC Press: Boca Raton, FL, USA, 2016; p. xiii. 394p. [Google Scholar]
- Schreiber, T.; Schmitz, A. Improved Surrogate Data for Nonlinearity Tests. Phys. Rev. Lett. 1996, 77, 635–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Mario, A.; Arakaki, T.; Arillo, V.C.; Chana, P.; Fernandez, W.; Garretto, N.; Martinez-Castrillo, J.C.; Rodriguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingwell, J.B.; Cusumano, J.P. Nonlinear time series analysis of normal and pathological human walking. Chaos 2000, 10, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.S.; Rintala, D.H.; Hou, J.G.; Collins, R.L.; Protas, E.J. Gait variability in Parkinson’s disease: Levodopa and walking direction. Acta Neurol. Scand. 2016, 134, 83–86. [Google Scholar] [CrossRef]
- Kurz, M.J.; Hou, J.G. Levodopa influences the regularity of the ankle joint kinematics in individuals with Parkinson’s disease. J. Comput. Neurosci. 2010, 28, 131–136. [Google Scholar] [CrossRef]
- Malek, N.; Kanavou, S.; Lawton, M.A.; Pitz, V.; Grosset, K.A.; Bajaj, N.; Barker, R.A.; Ben-Shlomo, Y.; Burn, D.J.; Foltynie, T.; et al. L-dopa responsiveness in early Parkinson’s disease is associated with the rate of motor progression. Parkinsonism Relat. Disord. 2019, 65, 55–61. [Google Scholar] [CrossRef]
- Thanvi, B.; Lo, N.; Robinson, T. Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad Med. J. 2007, 83, 384–388. [Google Scholar] [CrossRef]



| ID | Age | Height | Mass | BMI | MDS-UPDRS-III | LEDD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (yrs) | (m) | (kg) | (kg/m2) | TP1 | TP2 | TP3 | TP1 | TP2 | TP3 | |
| PD1 | 57.6 | 1.78 | 81.8 | 25.8 | 25 | 19 | 30 | 580 | 865 | 1081 |
| PD2 | 61.3 | 1.73 | 72.8 | 24.3 | 37 | 43 | 32 | 220 | 300 | 600 |
| PD3 | 65.2 | 1.76 | 112.4 | 36.3 | 33 | 34 | 26 | 730 | 483 | 2031 |
| PD4 | 80.1 | 1.72 | 68.6 | 23.2 | 36 | 39 | 37 | 475 | 575 | 575 |
| PD5 | 76.5 | 1.74 | 89.8 | 29.7 | 28 | 41 | 41 | 300 | 400 | 500 |
| Mean (SD) | 68.1 (9.7) | 1.74 (0.02) | 85.1 (17.3) | 27.9 (5.3) | 32(5) | 35 (10) | 33 (6) | 461 (207) | 525 (216) | 957.4 (643) |
| HOA1 | 77.0 | 1.75 | 76.4 | 24.9 | - | - | - | - | - | - |
| HOA2 | 61.6 | 1.75 | 83.4 | 27.2 | - | - | - | - | - | - |
| HOA3 | 73.8 | 1.84 | 110.6 | 32.7 | - | - | - | - | - | - |
| HOA4 | 69.6 | 1.76 | 80.6 | 27.9 | - | - | - | - | - | - |
| HOA5 | 84.0 | 1.74 | 82.0 | 27.1 | - | - | - | - | - | - |
| Mean (SD) | 73.2(8.3) | 1.77(0.04) | 86.6(13.7) | 28.0(2.9) | - | - | - | - | - | - |
| Group | Time (Months) | Total Strides | Strides Per Bout | Stride Time (S) |
|---|---|---|---|---|
| PD | 0 | 7244 | 29.7 ± 7.8 | 1.31 ± 0.21 |
| PD | 18 | 8502 | 30.7 ± 6.9 | 1.27 ± 0.17 |
| PD | 36 | 9473 | 31.7 ± 7.8 | 1.26 ± 0.17 |
| HOA | 0 | 7563 | 23.3 ± 5.2 | 0.93 ± 0.11 |
| HOA | 18 | 10386 | 29.9 ± 7.2 | 1.28 ± 0.16 |
| HOA | 36 | 9379 | 29.0 ± 7.1 | 1.31 ± 0.18 |
| Group | Time Series | p-Value * | Surrogate Time Series | p-Value ** |
|---|---|---|---|---|
| PD | 0.65 ± 0.09 | 0.008 | 1.31 ± 0.06 | 5.95 × 10−5 |
| HOA | 0.55 ± 0.11 | 1.27 ± 0.09 | 5.95 × 10−5 |
| ID | TP1 | TP2 | TP3 | |||
|---|---|---|---|---|---|---|
| N | SampEnt | N | SampEnt | N | SampEnt | |
| PD1 | 5618 | 0.68 | 11,647 | 0.65 | 10,721 | 0.97 |
| PD2 | 6844 | 0.55 | 4691 | 0.61 | 7878 | 0.62 |
| PD3 | 6800 | 0.65 | 6860 | 0.79 | 6761 | 1.06 |
| PD4 | 7988 | 0.60 | 9668 | 0.53 | 12,999 | 0.50 |
| PD5 | 8970 | 0.60 | 9646 | 0.69 | 9007 | 0.79 |
| Mean± SD | 7244± 1278 | 0.61± 0.05 | 8502± 2729 | 0.65± 0.09 | 9473.2± 2455 | 0.79± 0.24 |
| HOA1 | 7574 | 0.27 | 13366 | 0.36 | 12094 | 0.46 |
| HOA2 | 6545 | 0.57 | 8088 | 0.53 | 7204 | 0.45 |
| HOA3 | 8716 | 0.59 | 9527 | 0.61 | 10081 | 0.62 |
| HOA4 | 8179 | 0.46 | 9156 | 0.58 | 7208 | 0.71 |
| HOA5 | 6805 | 0.71 | 11792 | 0.51 | 10308 | 0.51 |
| Mean± SD | 7564 ± 911 | 0.52 ± 0.17 | 10386± 2144 | 0.52 ± 0.10 | 9379 ± 2131 | 0.55 ± 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coates, L.; Shi, J.; Rochester, L.; Del Din, S.; Pantall, A. Entropy of Real-World Gait in Parkinson’s Disease Determined from Wearable Sensors as a Digital Marker of Altered Ambulatory Behavior. Sensors 2020, 20, 2631. https://0-doi-org.brum.beds.ac.uk/10.3390/s20092631
Coates L, Shi J, Rochester L, Del Din S, Pantall A. Entropy of Real-World Gait in Parkinson’s Disease Determined from Wearable Sensors as a Digital Marker of Altered Ambulatory Behavior. Sensors. 2020; 20(9):2631. https://0-doi-org.brum.beds.ac.uk/10.3390/s20092631
Chicago/Turabian StyleCoates, Lucy, Jian Shi, Lynn Rochester, Silvia Del Din, and Annette Pantall. 2020. "Entropy of Real-World Gait in Parkinson’s Disease Determined from Wearable Sensors as a Digital Marker of Altered Ambulatory Behavior" Sensors 20, no. 9: 2631. https://0-doi-org.brum.beds.ac.uk/10.3390/s20092631






