Examination of Spectral Properties of Medicinal Plant Leaves Grown in Different Lighting Conditions Based on Mint Cultivation
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental
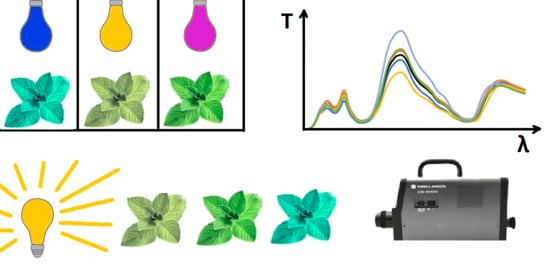
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yahia, I.B.H.; Jaouadi, R.; Trimech, R.; Boussaid, M.; Zaouali, Y. Variation of chemical composition and antioxidant activity of essential oils of Mentha x rotundifolia (L.) Huds. (Lamiaceae) collected from different bioclimatic areas of Tunisia. Biochem. Syst. Ecol. 2019, 84, 8–16. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Jadczak, D. Estimation of biological value of some species of mint (Mentha L.). Herba Pol. 2009, 55, 193–199. [Google Scholar]
- Balakrishnan, A. Therapeutic Uses of Peppermint—A Review. J. Pharm. Sci. Res. 2015, 7, 474–476. [Google Scholar]
- Derwich, E.; Benziane, Z.; Taouil, R.; Senhaji, O.; Touzani, M. Aromatic plants in Morocco: GC/MS analysis of essential oils of leaves of Mentha piperita. Adv. Environ. Biol. 2010, 4, 80–85. [Google Scholar]
- Eteghad, S.S.; Mirzaei, M.; Pour, S.F.; Kahnamui, S. Inhibitory Effects of endemic Thymus vulgaris and Mentha piperita essential oils on Escherichia coli O157:H7. Res. J. Biol. Sci. 2009, 4, 340–344. [Google Scholar]
- Kizil, S.; Hasimi, N.; Tolan, V.; Kilinc, E. Mineral content, essential oil components and biological activity of two mentha species (M. piperita L., M. spicata L.). Turk. J. Field Crops 2010, 2, 148–153. [Google Scholar]
- Kałwa, K.; Wilczyński, K.; Olesińska, K. Wpływ warunków przechowywania suszonej Mięty pieprzowej (Mentha piperita L.) na antyoksydacyjne właściwości otrzymanych naparów oraz zawartość i skład olejku eterycznego (The effect of storage conditions of dried Peppermint Mentha piperita L. on the antioxidant properties of the infusions obtained and the content and composition of essential oils. Acta Sci. Pol. Tech. Agrar. 2017, 16, 13–22. [Google Scholar]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N. Plant Growth and Physiological Responses to Light Conditions. In Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 2.2. [Google Scholar]
- Cegielski, T.; Bujalski, D.; Kowalczyk, K.; Gajc-Wolska, J.; Hemka, L. Use of light emission programming in tomato grow light system. Proc. Electrotech. Inst. 2016, 63, 79–94. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Economic Analysis of Greenhouse Lighting: Light Emitting Diodes vs. High Intensity Discharge Fixtures. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- MacCree, K.J. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Faline, D.M.; Plantenga, S.W.; Bergonzi, C.; Bachem, W.B. High light accelerates potato flowering independently of the FT-like flowering signal StSP3D. Environ. Exp. Bot. 2019, 160, 35–44. [Google Scholar]
- Christiaensac, A.; Gobina, B.; Huylenbroeckb, J.V. Adventitious rooting of Chrysanthemum is stimulated by a low-red:far-red ratio. J. Plant Physiol. 2019, 236, 117–123. [Google Scholar] [CrossRef]
- Meng, M.; Runkle, E.S. Growth Responses of Red-Leaf Lettuce to Temporal Spectral Changes. Front. Plant Sci. 2020, 11, 1623. [Google Scholar] [CrossRef]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous Irradiation with Alternating Red and Blue Light Enhances Plant Growth While Keeping Nutritional Quality in Lettuce. Hort. Sci. 2018, 53, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Xu, K. Silicon electro-optic micro-modulator fabricated in standard CMOS technology as components for all silicon monolithic integrated optoelectronic systems. Micromech. Microeng. 2021, 31. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Y.; Okhai, T.A.; Snyman, L.W. Micro optical sensors based on avalanching silicon light- emitting devices monolithically integrated on chips. Opt. Mat. Expr. 2019, 9, 3985. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory. An Indoor Vertical Farming System for Efficient Quality Food Production; Elsevier Inc.: San Diego, Waltham, USA, 2020. [Google Scholar]
- Hoang, H.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Development of an in vitro hydroponic culture system for wasabi nursery plant production—Effects of nutrient concentration and supporting material on plantlet growth. Sci. Hortic. 2019, 245, 237–243. [Google Scholar] [CrossRef]
- Mottier, P. LEDs for Lighting Applications; Willey: Hoboken, NJ, USA, 2009. [Google Scholar]
- DiLaura, D.L.; Houser, K.W.; Mistrick, R.G.; Steffy, G.R. The Lighting Handbook; Illuminating Engineering Society: New York, NY, USA, 2011. [Google Scholar]
- Measurement of LEDs; Publication No. 127; Central Bureau of the CIE: Vienna, Austria, 2007.
- Mazikowski, A.; Feldzensztajn, M. Lamp of adjustable spectrum for photographic usage. Proc. SPIE 2017, 10445. [Google Scholar] [CrossRef]
- Kington, N.; Bugbee, B. A Mixture of Barilium Sulfateand White Paint is a Low-Cost Substitute Reflectance Standard for Spectralon, Utah State University. 2005. Available online: http://www.triticeaecap.org/wp-content/uploads/2011/12/Barium_Sulfate.pdf (accessed on 15 June 2021).
- Chen, X.L.; Wang, L.C.; Li, T.; Yang, Q.C.; Guo, W.Z. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef] [Green Version]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 264–266. [Google Scholar] [CrossRef]
- Inoue, F.; Sugiura, H.; Tabuchi, A.; Karasawa, D.; Minami, M. Plant Regeneration of Peppermint, Mentha plperlta from the Hairy Roots Generated from Microsegment Infected with Agrobacterium rhizogenes. Plant Biotechnol. 2003, 20, 169. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Dembele, D.; Keller, E.R. Influence of explant, temperature and different culture vessels on in vitro culture for germplasm maintenance of four mint accessions. Plant Cell Tissue Organ Cult. 2005, 81, 123. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient Management in Aquaponics: Comparison of Three Approaches for Cultivating Lettuce, Mint and Mushroom Herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Litvin, A.G.; Wilson, L.A.; Currey, C.J. Effects of supplemental light source on basil, dill, and parsley growth, morphology, aroma, and flavor. J. Am. Soc. Horticult. Sci. 2020, 145, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.C.; Ratner, K.; Eidelman, O.; Bednarczyk, D.; Zur, N.; Many, Y.; Shahak, Y.; Aviv-Sharon, E.; Achiam, M.; Gilad, Z.; et al. Effects of daytime intra-canopy LED illumination on photosynthesis and productivity of bell pepper grown in protected cultivation. Sci. Hortic. 2019, 250, 81–88. [Google Scholar] [CrossRef]
- Metallo, R.M.; Kopsell, D.A.; Sams, C.E.; Bumgarner, N.R. Influence of blue/red vs. white LED light treatments on biomass, shoot morphology, and quality parameters of hydroponically grown kale. Sci. Hortic. 2018, 235, 189–197. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. Chinensis. J. Photochem. Photobiol. B Biol. 2019, 193, 18–30. [Google Scholar] [CrossRef]
- Gautier, H.; Rocci, A.; Buret, M.; Grasselly, D.; Dumas, Y.; Causse, M. Effect of photoselective filters on the physical and chemical traits of vine-ripened tomato fruits. Can. J. Plant Sci. 2005, 85, 439–446. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato Grafting: A Global Perspective. HortSci. Horts 2017, 52, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Croser, J.S.; Pazos-Navarro, M.; Bennett, R.G.; Tschirren, S.; Edwards, K.; Erskine, W.; Creasy, R.; Ribalta, F.M. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tiss Organ Cult. 2016, 127, 591–599. [Google Scholar] [CrossRef]
- Haining, R.R.; Bimber, O. Displays; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Luo, M.R.; Cui, G.; Rigg, B. The Development of the CIE 2000 Colour-Difference Formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Rodrigues Cruz, K.; Materón Vásques, E.M.; Novais de Oliveira, O., Jr. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for eHealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [Green Version]





| Light Source | Lamp 1 | Lamp 2 | Lamp 3 | Lamp 4 | Lamp 5 | Lamp 6 |
|---|---|---|---|---|---|---|
| PPFD [µmol/m2/s] | 165.5 | 166.7 | 167.6 | 167.1 | 167 | 167.1 |
| Light Sources | Lamp 1 | Lamp 2 | Lamp 3 | Lamp 4 | Lamp 5 | Lamp 6 |
|---|---|---|---|---|---|---|
| Lamp 1 | - | 3.55 | 2.67 | 8.35 | 2.94 | 3.73 |
| Lamp 2 | 3.55 | - | 1.03 | 6.76 | 8.12 | 1.88 |
| Lamp 3 | 2.67 | 1.03 | - | 5.10 | 3.93 | 2.43 |
| Lamp 4 | 8.35 | 6.76 | 5.10 | - | 15.37 | 3.20 |
| Lamp 5 | 2.94 | 8.12 | 3.93 | 15.37 | - | 9.90 |
| Lamp 6 | 3.73 | 1.88 | 2.43 | 3.20 | 9.90 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldzensztajn, M.; Wierzba, P.; Mazikowski, A. Examination of Spectral Properties of Medicinal Plant Leaves Grown in Different Lighting Conditions Based on Mint Cultivation. Sensors 2021, 21, 4122. https://0-doi-org.brum.beds.ac.uk/10.3390/s21124122
Feldzensztajn M, Wierzba P, Mazikowski A. Examination of Spectral Properties of Medicinal Plant Leaves Grown in Different Lighting Conditions Based on Mint Cultivation. Sensors. 2021; 21(12):4122. https://0-doi-org.brum.beds.ac.uk/10.3390/s21124122
Chicago/Turabian StyleFeldzensztajn, Mateusz, Paweł Wierzba, and Adam Mazikowski. 2021. "Examination of Spectral Properties of Medicinal Plant Leaves Grown in Different Lighting Conditions Based on Mint Cultivation" Sensors 21, no. 12: 4122. https://0-doi-org.brum.beds.ac.uk/10.3390/s21124122