Fiber Optic Gas Sensors Based on Lossy Mode Resonances and Sensing Materials Used Therefor: A Comprehensive Review
Abstract
:1. Introduction
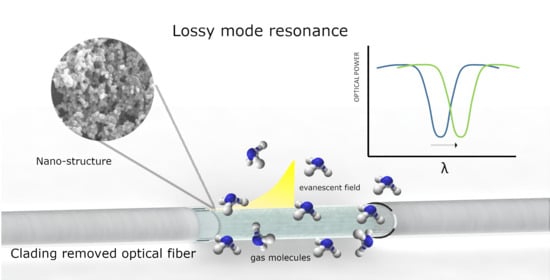
2. Fundamentals of Lossy Mode Resonances (LMR)
3. Ammonia Sensors
| Sensing Layer | Deposition Technique | Optical Waveguide | Gas Concentration | Sensitivity | Detection Limit | Response Time | Recovery Time | Reference |
|---|---|---|---|---|---|---|---|---|
| MPyP/TiO2 | Liquid phase deposition method | taper of doped optical fibre (SM750) | 0.1 to 10,000 ppm | Nonlinear ~1.2 nm ppm−1 in the range 0.1–10 ppm. | 0.16 ppm | 30 s | N/A | [46] |
| Magnesium tetraborate, MgB4O7 (MTB) | Slurry deposition | PMMA 750 µm | 0 to 500 ppm | 1.48 counts ppm−1 | 50 ppm * | N/A | N/A | [47] |
| CeO2/Multi walled carbon nanotubes | Hydrothermal technique | PMMA 550 µm | 0 to 500 ppm | 0.096 counts ppm−1 | 50 ppm * | 28 s | 19 s | [56] |
| Three-dimensional ZnO nanoflowers | Drop coating | SM-MM-SM | 0 to 7281 ppm | 4.32 pm ppm−1 | 1210 ppm * | ~26 s | ~39 s | [58] |
| ZnO nanostructures | Pulsed laser deposition method | D-shape fiber | 0 to 5000 ppm | ~0.66 pm ppm−1 | ~50 ppm | N/A | N/A | [59] |
| Graphene oxide | Dip coating | PMMA 750 µm | 0 to 500 ppm | −0.32 counts ppm−1 | 100 ppm * | N/A | N/A | [61] |
| Nanocrystalline ZnO | Dip coating | PMMA 750 µm | 0 to 14 KPa | − 0.017 kPa−1 | 0.2 kPa * | 100 min | 80 min | [62] |
| Nanocrystalline CeO2 | Dip coating | PMMA 750 µm | 0 to 14 KPa | 12 10−3 kPa−1 | 0.2 kPa * | 64 min | 51 min | [63] |
| Nanoparticles of V2O5 and WO3 | Dip coating | PMMA 750 µm | 0 to 14 KPa | 4 10−3 kPa−1 | 0.2 kPa * | 40 min | 39 min | [64] |
| Nanocrystalline SnO2 and fim SnO2 | Thermal evaporation | PMMA 700 µm | 100 to 1000 ppm | 0.09 counts ppm−1 | 100 ppm * | 20 min | 120 min | [65] |
| Nanocrystalline TiO2 | Dip coating | PMMA 750 µm | 50 to 500 ppm | 0.6 counts ppm−1 | 50 ppm * | 83 min | 77 min | [20] |
| Zn3(VO4)2 nanopowder | Dip coating | PMMA 750 µm | 20 to 500 ppm | 0.019 µV ppm−1 | 50 ppm * | 46.8 min | 59 min | [66] |
| Sm2O3 | Dip coating | PMMA 750 µm | 0 to 500 ppm | 14 10−3 kPa−1 | 1 kPa * | 67 min | 57 min | [67] |
| Single and multi-walled carbon nanotubes | Dip coating | PMMA 750 µm | 0 to 500 ppm | 0.31 counts ppm−1 | 50 ppm * | N/A | N/A | [68] |
| Single-walled carbon nanotubes | Dip coating | PMMA 750 µm | 0 to 500 ppm | 1.3 counts ppm−1 | 12.5 ppm * | 60 min | 50 min | [69] |
4. Humidity Sensors
| Sensing Layer | Deposition Technique | Optical Waveguide | Sensitivity | Response Time | Recovery Time | Reference |
|---|---|---|---|---|---|---|
| Indium tin oxide (ITO) and Polyallylamine hydrochloride (PAH) and polyacrylic acid (PAA) | Dip coating and Layer-by-Layer | Optical fiber 225/200 μm | 0.82 nm/%RH | approx. 1870 s | approx. 5250 s | [77] |
| FT200EMT | ||||||
| Indium tin oxide (ITO) and agarose | Dip coating and Boiling water method | Optical fiber 225/200 μm | 0.75 nm/%RH | N/A | N/A | [79] |
| FT200EMT | ||||||
| Tin oxide (SnO2) | Dip coating | Optical fiber 225/200 μm | 0.107 nm/%RH | N/A | N/A | [80] |
| FT200EMT | ||||||
| Polyallylamine hydrochloride (PAH) and polyacrylic acid (PAA) | Layer-by-Layer | Optical fiber 225/200 μm | 0.37 nm/%RH | N/A | N/A | [81] |
| FT200EMT | ||||||
| TiO2 and poly(sodium 4-styrenesulfonate) (PSS) | Layer-by-Layer | Optical fiber 225/200 μm | 1.43 nm/%RH for 1° resonance 0.97 nm/%RH for 2° | N/A | N/A | [78] |
| FT200EMT | ||||||
| Polyallylamine hydrochloride (PAH) and polyacrylic acid (PAA) | Layer-by-Layer | Optical fiber 225/200 μm | 0.51 nm/%RH First LMR | N/A | N/A | [28] |
| FT200EMT | ||||||
| Polymeric and Ag particles | Layer-by-Layer | Optical fiber 225/200 μm | 0.943 nm/%RH | 0.476 s | 0.447 s | [84] |
| FT200EMT | ||||||
| Indium tin oxide (ITO) and In2O3 | Layer-by-Layer | Optical fiber 225/200 μm | 0.283, 0.133, 0.042 nm/%RH ITO, In2O3 TE and TM mode | N/A | N/A | [85] |
| FT200EMT | ||||||
| Tin oxide (SnO2) | Sputtering | Taper SMF 9/125 um Telnet RI | 1.9 nm/%RH | 1.52 s | approx. 1.52 s | [86] |
| Indium tin oxide (ITO) | Sputtering | Planar waveguide coverslips | 0.0657 nm/%RH from 30 to 65%RH | N/A | N/A | [87] |
| 0.212 nm/%RH from 65 to 90%RH | ||||||
| SnO2 polyethylenimine (PEI) and graphene oxide (GO) | Layer-by-layer and sputtering | Optical fiber 225/200 μm | 0.317 nm/%RH from 20 to 70%RH | 0.16 s | 0.262 s | [24] |
| FT200EMT | 0.212 nm/%RH from 65 to 90%RH | |||||
| Polymeric film and gold nanorods | Layer-by-Layer | Optical fiber 225/200 μm | ~3 nm/%RH from 20 to 45%RH | N/A | N/A | [88] |
| FT200EMT | 11.2 nm/%RH from 45 to 90%RH | |||||
| Graphene oxide (GO) | Spontaneous evaporation | Type D single mode fiber | 0.145 nm/%RH from 32 to 85%RH | 2.73 s | 7.27 s | [44] |
| 0.915 nm/%RH from 85 to 97.6%RH | ||||||
| Copper oxide (CuO), tin oxide (SnO2) and agarose | DC sputtering and boiling water method | Coverslips | 0.636 nm/%RH from 30 to 90%RH | N/A | N/A | [89] |
5. Volatile Compounds (VCs)
| Gas ** | Sensing Layer | Deposition Technique | Gas Concentration | Sensitivity | Detection Limit | Optical Waveguide | Ref. |
|---|---|---|---|---|---|---|---|
| (2), (4), (1) | Nanocrystalline TiO2 | Dip coating | 50 to 500 ppm | 0.6 (2), 0.35 (4), 0.29 (1) counts ppm−1 | 50 ppm * | Poly(methyl methacrylate) PMMA 750 µm | [20] |
| (1), (2) | CeO2/Multi walled carbon nanotubes | Hydrothermal technique | 0 to 500 ppm | 0.158 (1), 0.096 (2) counts ppm−1 | 50 ppm * | PMMA 550 µm | [56] |
| (2), (1), (4) | Graphene oxide | Dip coating | 0 to 500 ppm | −0.32 (2), -0.26 (1), −0.2 (4) counts ppm−1 | 100 ppm * | PMMA 750 µm | [61] |
| (4), (1), (2) | Nanocrystalline ZnO | Dip coating | 0 to 14 (2), 2.5 (1), 1.4 (4) kPa | 210 (4), 190 (1), −170 (2) 10−3 kPa−1 | ~ 0.2 kPa * | PMMA 750 µm | [62] |
| (4), (1), (2) | Nanocrystalline CeO2 | Dip coating | 0 to 14 (2), 2.5 (1), 1.4 (4) kPa | 71 (4), 12 (2), 5 (1) 10−3 kPa−1 | ~ 0.2 kPa * | PMMA 750 µm | [63] |
| (4), (1), (2) | Nanoparticles of V2O5 (a) and WO3 (b) | Dip coating | 0 to 14 (2), 2.5 (1), 1.4 (4) kPa | (a): 56 (4), 4 (2) 10−3 kPa−1(b): 59 (4), 5 (2), 4 (1) 10−3 kPa−1 | ~ 0.2 kPa * | PMMA 750 µm | [64] |
| (2), (1), (4), (3) | Zn3(VO4)2 nanopowder | Dip coating | 20 to 500 ppm | 0.19 (2), 0.009 (1), 0.005 (4), 0.004 (3) µV ppm−1 | 50 ppm * | PMMA 750 µm | [66] |
| (2), (4), (1) | Sm2O3 | Dip coating | 0 to 14 (2), 2.5 (1), 1.4 (4) kPa | 14 (2), 8 (4), 76 (1) 10−3 kPa−1 | ~1 kPa * (2) | PMMA 750 µm | [67] |
| ~0.75 kPa * (1) | |||||||
| ~0.5 kPa * (4) | |||||||
| (2), (1) | Single-walled carbon nanotubes | Dip coating | 0 to 500 ppm | 1.3 (2) 1.12 (1) counts ppm−1 | 12.5 ppm * | PMMA 750 µm | [69] |
| (1) | SnO2 (a) and SnO2: CuO (b) | Co-precipitation | 0 to 500 ppm | −0.13 (a), −0.41 (b) counts ppm−1 | 50 ppm * | N/A | [91] |
| (3) | Magnesium tetraborate (MTB) (a), MTB doped with cerium (b), MTB doped with cerium after gamma irradiation (c) | Slurry deposition | 50 to 500 ppm | 0.04 (a), 0.54 (b), 0.7 (c) counts ppm −1 | 50 ppm * | PMMA 750 µm | [92] |
| (1) | WO3/gC3N4 (a) and WO3 (b) nanocomposites | Dip coating | 0 to 500 ppm | 62.5 (a), 38.5 (b) % at 500 ppm | 100 ppm * | PMMA 550 µm | [97] |
| (4), (9), (1) | Organometallic compound [Au2Ag2(C6F5)4(NH3)2] n | Layer-by-Layer | 0 to 250 ppm | 0.131 (4), 0.074 (9), 0.067 (1) nm ppm−1 | 25 ppm * | Plastic cad silica PCS fiber | [98] |
| (4) | (PAH/SiO2)n and (TPPS) | Layer-by-Layer | N/A | N/A | N/A | U-shaped PCS 200 µm | [99] |
| (6) | Polypyrrole/Prussian blue/Titanium dioxide composite (PPy/PB/TiO2) (a) and TiO2 (b) | Dip coating | 0 to 500 ppm | 1.51 (a), 0.78 (b) counts ppm −1 | 50 ppm * | PMMA 750 µm | [100] |
| (1), (4), (5) | ZnO Nanorods | Dip coating | 0 to 250 ppm | ~6.5% (1), ~3% (4), ~1.2% (5) at 300 ppm | 50 ppm * | PMMA 1000 µm | [101] |
| (1), (3) | Nanocrystalline MnCo2O4 | Dip coating | 0 to 500 ppm | 0.11 (1), 0.068 (3) counts ppm−1 | 50 ppm * | PMMA 550 µm | [102] |
| (3), (2), (1) | Pristine (a) and amine functionalized (b) ZnO nanoflake | Dip coating | 0 to 300 ppm | (a): 13 (3), 7 (2), 7 (5), 4 (1), 1.8 (7), 1.8 (4) % | 30 (3), 20 (2), 10 (1), 10 (4), 10 (10), 10 (7) ppm | PMMA 1 mm | [103] |
| (4), (5), (7) | (b): 45 (2), 18 (7), 18 (5), 15 (3), 8 (1), 8 (4) % | ||||||
| (3), (2), (1) | Nanocrystalline copper bromide (γ-CuBr) | Slurry deposition | 0 to 750 ppm | 5% (3), 0.75% (1), 0.42% (2) with 750 ppm | ~50 ppm * | PMMA 750 µm | [104] |
| (3), (2), (1) | ZnO nanorhombuses | Dip coating | 0 to 400 ppm | ~24% (3), ~12% (2), ~4% (1) thiourea functionalized at 772 nm and 400 ppm | 10 ppm * | PMMA 1000 µm | [105] |
| (3), (2), (1) | ZnO nanoparticles | No data | 0 to 250 ppm | 14% (3), 9% (2), 5% (1) with 250 ppm | 50 ppm * | no data found | [106] |
| (3), (6), (8) | TiO2 | Dip coating | 0 to 500 ppm | −0.31 (3), −0.15 (6), −0.12 (8) counts ppm−1 | 50 ppm * | PMMA 750 µm | [107] |
| (1) | Nano-sized amorphous tin oxide SnO2 | Electron-beam evaporation | 1000 to 20,000 ppm | 14% at 10,000 ppm | 1000 ppm * | SMF | [108] |
| (1) | Undoped (a), Mn-doped (b) cobal ferrite CoFe2O4 nanoparticles | Chemical method | 0 to 500 ppm | 0.07 (a), 0.12 (b) counts ppm−1 | 100 ppm * | PMMA 750 µm | [109] |
| (4), (1), (2) | ZnO (a), Ce doped Zno (b), Al2O3 (c), CeO2 (d), Sm2O3 (e), WO3 (f), V2O5(g) | Chemical method | 0 to 500 ppm | (2): −17 (a), 7 (b), 9 (c), 12 (d), 14 (e), −5 (f), 4 (g) 10−3 kPa−1 | N/A | PMMA | [110] |
| (4): 21 (a), −96 (b), 29 (c), 71 (d), 8 (e), 59 (f), 56 (g) 10−3 kPa−1 | |||||||
| (1): −21 (a), −131 (b), 55 (c), −5 (d), 76 (e), −4 (f), 1 (g) 10−3 kPa−1 | |||||||
| (1) | Gd doped ZnO nanoparticles | Dip coating | 0 to 1000 ppm | 0.0552 counts ppm−1 | 100 ppm * | PMMA 750 µm | [111] |
| (1), (4), (9) | Organometallic compound [Au2Ag2(C6F5)4(NH3)2]n | Layer-by-Layer | 0 to 140 (1), 150 (4), 200 (9) ppm | 0.41 (1), 0.52 (4), 0.263(9) nm ppm−1 | 4 (1), 16 (4), 10 (9) ppm | PCS 200/220 um | [112] |
| (1), (4), (9) | Lithium tetraborate Li2B4O7 | Gel method | 50 to 500 ppm | −10 (1), −4 (4), 3 (2) counts ppm−1 | 50 ppm | PMMA 750 µm | [113] |
| (1) | Chunk-shaped ZnO nanoparticles | Thermal evaporation | 0 to 500 ppm | 3.5% for 500 ppm | 100 ppm * | PMMA 700 µm | [114] |
| (3), (6), (8) | Nano-crystalline zinc oxide ZnO | Dip coating | 0 to 500 ppm | 0.009 (3), −0.001 (6), −0.0017 (8) counts ppm−1 | 50 ppm * | PMMA 750 µm | [115] |
6. Other Gases
6.1. Hydrogen Sulfide (H2S) Sensors
6.2. Hydrogen H2 Sensors
6.3. NOX Sensors
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed Abdul-Wahab, S.A.; En, S.C.F.; Elkamel, A.; Ahmadi, L.; Yetilmezsoy, K. A review of standards and guidelines set by international bodies for the parameters of indoor air quality. Atmos. Pollut. Res. 2015, 6, 751–767. [Google Scholar] [CrossRef]
- Ghobakhloo, M. Industry 4.0, digitization, and opportunities for sustainability. J. Clean. Prod. 2020, 252, 119869. [Google Scholar] [CrossRef]
- Cavicchi, R.E. Calorimetric Sensors. In Chemical Sensors; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011; pp. 287–320. ISBN 978-1-60650-233-4. [Google Scholar]
- Haghighi, F.; Talebpour, Z.; Sanati-Nezhad, A. Through the years with on-a-chip gas chromatography: A review. Lab Chip 2015, 15, 2559–2575. [Google Scholar] [CrossRef]
- Guiochon, G.; Guillemin, C.L. Gas chromatography. Rev. Sci. Instrum. 1990, 61, 3317–3339. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Zhu, M.; Zhang, K.; Liu, T.; Xu, D. Acoustic absorption spectral peak location for gas detection. Sens. Actuators B Chem. 2014, 203, 1–8. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef] [Green Version]
- Pawar, D.; Kale, S.N. A review on nanomaterial-modified optical fiber sensors for gases, vapors and ions. Microchim. Acta 2019, 186, 253. [Google Scholar] [CrossRef]
- Campanella, C.E.; Cuccovillo, A.; Campanella, C.; Yurt, A.; Passaro, V.M.N. Fibre Bragg Grating based strain sensors: Review of technology and applications. Sensors 2018, 18, 3115. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, J.; Zhao, Q. Review of no-core optical fiber sensor and applications. Sens. Actuators A Phys. 2020, 313, 112160. [Google Scholar] [CrossRef]
- Korposh, S.; James, S.W.; Lee, S.W.; Tatam, R.P. Tapered Optical Fibre Sensors: Current Trends and Future Perspectives. Sensors 2019, 19, 2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, M.K. D-Type Optical Fiber & its Applications. Int. J. Sci. Eng. Res. 2014, 5, 952–955. [Google Scholar]
- Xu, H.; Wu, P.; Zhu, C.; Elbaz, A.; Gu, Z.Z. Photonic crystal for gas sensing. J. Mater. Chem. C 2013, 1, 6087–6098. [Google Scholar] [CrossRef]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Khan, S.; Le Calvé, S.; Newport, D. A review of optical interferometry techniques for VOC detection. Sens. Actuators A Phys. 2020, 302, 111782. [Google Scholar] [CrossRef]
- Zhao, X.W.; Wang, Q. Mini review: Recent advances in long period fiber grating biological and chemical sensors. Instrum. Sci. Technol. 2019, 47, 140–169. [Google Scholar] [CrossRef]
- Tabassum, R.; Kant, R. Recent trends in surface plasmon resonance based fiber–optic gas sensors utilizing metal oxides and carbon nanomaterials as functional entities. Sens. Actuators B Chem. 2020, 310, 127813. [Google Scholar] [CrossRef]
- Jorgenson, R.C.; Yee, S.S. A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Actuators B Chem. 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Gobi, G.; Srinivasan, R.; Rajeshwari Yogamalar, N.; Chandra Bose, A. Nanocrystalline Titanium dioxide coated optical fiber sensor for ammonia vapour detection. Nanoeng. Fabr. Prop. Opt. Devices 2010, 7764, 77640U. [Google Scholar] [CrossRef]
- Okazaki, S.; Nakagawa, H.; Asakura, S.; Tomiuchi, Y.; Tsuji, N.; Murayama, H.; Washiya, M. Sensing characteristics of an optical fiber sensor for hydrogen leak. Sens. Actuators B Chem. 2003, 93, 142–147. [Google Scholar] [CrossRef]
- Dreyer, U.J.; Ozcariz, A.; Ascorbe, J.; Zubiate, P.; Vitoria, I.; Martelli, C.; da Silva, J.C.C.; Zamarreño, C.R. Gas Detection Using LMR-Based Optical Fiber Sensors. Proceedings 2018, 2, 890. [Google Scholar] [CrossRef] [Green Version]
- Devendiran, S.; Sastikumar, D. Gas sensing based on detection of light radiation from a region of modified cladding (nanocrystalline ZnO) of an optical fiber. Opt. Laser Technol. 2017, 89, 186–191. [Google Scholar] [CrossRef]
- Hernaez, M.; Acevedo, B.; Mayes, A.G.; Melendi-Espina, S. High-performance optical fiber humidity sensor based on lossy mode resonance using a nanostructured polyethylenimine and graphene oxide coating. Sens. Actuators B Chem. 2019, 286, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Del Villar, I.; Zamarreño, C.R.; Hernaez, M.; Arregui, F.J.; Matias, I.R. Lossy mode resonance generation with indium-tin-oxide-coated optical fibers for sensing applications. J. Light. Technol. 2010, 28, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Del Villar, I.; Arregui, F.J.; Zamarreño, C.R.; Corres, J.M.; Bariain, C.; Goicoechea, J.; Elosua, C.; Hernaez, M.; Rivero, P.J.; Socorro, A.B.; et al. Optical sensors based on lossy-mode resonances. Sens. Actuators B Chem. 2017, 240, 174–185. [Google Scholar] [CrossRef]
- Ozcariz, A.; Dominik, M.; Smietana, M.; Zamarreño, C.R.; Del Villar, I.; Arregui, F.J. Lossy mode resonance optical sensors based on indium-gallium-zinc oxide thin film. Sens. Actuators A Phys. 2019, 290, 20–27. [Google Scholar] [CrossRef]
- Sanchez, P.; Zamarreno, C.R.; Hernaez, M.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Considerations for lossy-mode resonance-based optical fiber sensor. IEEE Sens. J. 2013, 13, 1167–1171. [Google Scholar] [CrossRef]
- Zubiate, P.; Zamarreño, C.R.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Tunable optical fiber pH sensors based on TE and TM Lossy Mode Resonances (LMRs). Sens. Actuators B Chem. 2016, 231, 484–490. [Google Scholar] [CrossRef]
- Usha, S.P.; Mishra, S.K.; Gupta, B.D. Fiber optic hydrogen sulfide gas sensors utilizing ZnO thin film/ZnO nanoparticles: A comparison of surface plasmon resonance and lossy mode resonance. Sens. Actuators B Chem. 2015, 218, 196–204. [Google Scholar] [CrossRef]
- Corres, J.M.; Ascorbe, J.; Arregui, F.J.; Matias, I.R. Tunable electro-optic wavelength filter based on lossy-guided mode resonances. Opt. Express 2013, 21, 31668. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Zubiate, P.; del Villar, I.; Zamarreño, C.R.; Giannetti, A.; Tombelli, S.; Trono, C.; Matias, I.R.; Arregui, F.J.; Baldini, F. Lossy mode resonance fiber-optic biosensing allowing ultra-low detection limit. Opt. InfoBase Conf. Pap. 2019, Part F140, 50019. [Google Scholar]
- Del Villar, I.; Hernaez, M.; Zamarreno, C.R.; Sánchez, P.; Fernández-Valdivielso, C.; Arregui, F.J.; Matias, I.R. Design rules for lossy mode resonance based sensors. Appl. Opt. 2012, 51, 4298–4307. [Google Scholar] [CrossRef] [PubMed]
- Del Villar, I.; Zamarreño, C.R.; Hernaez, M.; Arregui, F.J.; Matias, I.R. Resonances in coated long period fiber gratings and cladding removed multimode optical fibers: A comparative study. Opt. Express 2010, 18, 20183. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, O.; Del Villar, I.; Corres, J.M.; Matias, I.R. Lossy mode resonance sensors based on lateral light incidence in nanocoated planar waveguides. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Villar, I.; Torres, V.; Beruete, M. Experimental demonstration of lossy mode and surface plasmon resonance generation with Kretschmann configuration. Opt. Lett. 2015, 40, 4739. [Google Scholar] [CrossRef] [PubMed]
- Del Villar, I.; Zamarreño, C.R.; Sanchez, P.; Hernaez, M.; Valdivielso, C.F.; Arregui, F.J.; Matias, I.R. Generation of lossy mode resonances by deposition of high-refractive-index coatings on uncladded multimode optical fibers. J. Instrum. 2010, 5, 095503. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.; Franzen, S.; Maria, J.P.; Losego, M.; Leonard, D.N.; Laughlin, B.; Duscher, G.; Weibel, S. Surface plasmon resonance in conducting metal oxides. J. Appl. Phys. 2006, 100, 054905. [Google Scholar] [CrossRef] [Green Version]
- Del Villar, I.; Zamarreño, C.R.; Hernaez, M.; Arregui, F.J.; Matias, I.R. Generation of lossy mode resonances with absorbing thin-films. J. Light. Technol. 2010, 28, 3351–3357. [Google Scholar] [CrossRef] [Green Version]
- Ozcariz, A.; Zamarreño, C.R.; Zubiate, P.; Arregui, F.J. Is there a frontier in sensitivity with Lossy mode resonance (LMR) based refractometers? Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Sanchez, P.; Zamarreño, C.R.; Hernaez, M.; Matias, I.R.; Arregui, F.J. Optical fiber refractometers based on Lossy Mode Resonances by means of SnO2 sputtered coatings. Sens. Actuators B Chem. 2014, 202, 154–159. [Google Scholar] [CrossRef]
- Ozcariz, A.; Piña-Azamar, D.A.; Zamarreño, C.R.; Dominguez, R.; Arregui, F.J. Aluminum doped zinc oxide (AZO) coated optical fiber LMR refractometers—An experimental demonstration. Sens. Actuators B Chem. 2019, 281, 698–704. [Google Scholar] [CrossRef]
- Fuentes, O.; Vaiano, P.; del Villar, I.; Quero, G.; Corres, J.; Consales, M.; Matías, I.; Cusano, A. Improving the width of lossy mode resonances in a reflection configuration D-shaped fiber by nanocoating laser ablation. Opt. Lett. 2020, 45, 4738. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, W.; Li, Z.; Chen, G.; Chen, L.; Zhou, J.; Lin, H.; Guan, J.; Fang, W.; Liu, X.; et al. High-performance fibre-optic humidity sensor based on a side-polished fibre wavelength selectively coupled with graphene oxide film. Sens. Actuators B Chem. 2018, 255, 57–69. [Google Scholar] [CrossRef]
- Airoudj, A.; Debarnot, D.; Bêche, B.; Poncin-Epaillard, F. A new evanescent wave ammonia sensor based on polyaniline composite. Talanta 2008, 76, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Mullaney, K.; Korposh, S.; James, S.W.; Lee, S.W.; Tatam, R.P. An ammonia sensor based on Lossy Mode Resonances on a tapered optical fibre coated with porphyrin-incorporated titanium dioxide. Sens. Actuators B Chem. 2017, 242, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Mohandoss, R.; Renganathan, B.; Annalakshmi, O.; Ganesan, A.R. Fiber optics assisted ammonia gas detection property of gamma irradiated magnesium tetraborate. Sens. Actuators A Phys. 2019, 285, 158–164. [Google Scholar] [CrossRef]
- Korposh, S.; Kodaira, S.; Selyanchyn, R.; Ledezma, F.H.; James, S.W.; Lee, S.W. Porphyrin-nanoassembled fiber-optic gas sensor fabrication: Optimization of parameters for sensitive ammonia gas detection. Opt. Laser Technol. 2018, 101, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, P.P.; Zhao, J.; Feng, L.L.; Zhou, L.J.; Xuan, R.F.; Liu, Y.P.; Li, G.D. SnO2 nanoparticle-coated In2O3 nanofibers with improved NH3 sensing properties. Sens. Actuators B Chem. 2014, 194, 440–446. [Google Scholar] [CrossRef]
- Tao, L.; Sun, K.; Miller, D.J.; Pan, D.; Golston, L.M.; Zondlo, M.A. Low-power, open-path mobile sensing platform for high-resolution measurements of greenhouse gases and air pollutants. Appl. Phys. B Lasers Opt. 2015, 119, 153–164. [Google Scholar] [CrossRef]
- Xiong, L.; Compton, R.G. Amperometric gas detection: A review. Int. J. Electrochem. Sci. 2014, 9, 7152–7181. [Google Scholar]
- Constantinoiu, I.; Miu, D.; Viespe, C. Surface acoustic wave sensors for ammonia detection at room temperature based on SnO2/Co3O4 bilayers. J. Sens. 2019, 2019, 8203810. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.Y.; Chen, H.I.; Hsu, C.S.; Huang, C.C.; Chang, C.F.; Chou, P.C.; Liu, W.C. On an ammonia gas sensor based on a Pt/AlGaN heterostructure field-effect transistor. IEEE Electron. Device Lett. 2012, 33, 612–614. [Google Scholar] [CrossRef]
- Shanavas, S.; Ahamad, T.; Alshehri, S.M.; Sultan, A.; Acevedo, R.; Anbarasan, P.M. Development of high-performance fiber optic gas sensor based rice-like CeO2/MWCNT nanocomposite synthesized by facile hydrothermal route. Opt. Laser Technol. 2020, 123, 105902. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Fu, H.; Ding, J.; Li, H.; Zhang, M.; Zhang, J.; Liu, Y. Fabrication of three-dimensional zinc oxide nanoflowers for high-sensitivity fiber-optic ammonia gas sensors. Appl. Opt. 2018, 57, 7924. [Google Scholar] [CrossRef]
- Dikovska, A.O.; Atanasova, G.B.; Nedyalkov, N.N.; Stefanov, P.K.; Atanasov, P.A.; Karakoleva, E.I.; Andreev, A.T. Optical sensing of ammonia using ZnO nanostructure grown on a side-polished optical-fiber. Sens. Actuators B Chem. 2010, 146, 331–336. [Google Scholar] [CrossRef]
- Willmott, P.R. Deposition of complex multielemental thin films. Prog. Surf. Sci. 2004, 76, 163–217. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Sastikumar, D.; Manivannan, S. Effect of functional groups on dielectric, optical gas sensing properties of graphene oxide and reduced graphene oxide at room temperature. RSC Adv. 2015, 5, 10816–10825. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Gobi, G.; Rajeswari Yogamalar, N.; Chandra Bose, A. Nanocrystalline ZnO coated fiber optic sensor for ammonia gas detection. Opt. Laser Technol. 2011, 43, 1398–1404. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Bose, A.C.; Srinivasan, R.; Ganesan, A.R. Nanocrystalline cerium oxide coated fiber optic gas sensor. Curr. Appl. Phys. 2014, 14, 467–471. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Raj, S.G.; Ganesan, A.R. Fiber optic gas sensors with vanadium oxide and tungsten oxide nanoparticle coated claddings. Opt. Commun. 2014, 315, 74–78. [Google Scholar] [CrossRef]
- Renganathan, B.; Gobi, G.; Sastikumar, D.; Srinivasan, R.; Bose, A.C. Optical fiber coated with nanocrystalline tin oxide for ammonia vapour sensing. Sens. Lett. 2010, 8, 292–296. [Google Scholar] [CrossRef]
- Subramanian, M.; Dhayabaran, V.V.; Sastikumar, D.; Shanmugavadivel, M. Development of room temperature fiber optic gas sensor using clad modified Zn3 (VO4)2. J. Alloys Compd. 2018, 750, 153–163. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Srinivasan, R.; Ganesan, A.R. Nanocrystalline samarium oxide coated fiber optic gas sensor. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2014, 186, 122–127. [Google Scholar] [CrossRef]
- Manivannan, S.; Shobin, L.R.; Saranya, A.M.; Renganathan, B.; Sastikumar, D.; Park, K.C. Carbon nanotubes coated fiber optic ammonia gas sensor. Integr. Opt. Devices Mater. Technol. XV 2011, 7941, 79410M. [Google Scholar] [CrossRef]
- Manivannan, S.; Saranya, A.M.; Renganathan, B.; Sastikumar, D.; Gobi, G.; Park, K.C. Single-walled carbon nanotubes wrapped poly-methyl methacrylate fiber optic sensor for ammonia, ethanol and methanol vapors at room temperature. Sens. Actuators B Chem. 2012, 171–172, 634–638. [Google Scholar] [CrossRef]
- Brinker, C.J.; Hurd, A.J.; Schunk, P.R.; Frye, G.C.; Ashley, C.S. Review of sol-gel thin film formation. J. Noncryst. Solids 1992, 147–148, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.D.; Ratnanjali. A novel probe for a fiber optic humidity sensor. Sens. Actuators B Chem. 2001, 80, 132–135. [Google Scholar] [CrossRef]
- Khijwania, S.K.; Srinivasan, K.L.; Singh, J.P. An evanescent-wave optical fiber relative humidity sensor with enhanced sensitivity. Sens. Actuators B Chem. 2005, 104, 217–222. [Google Scholar] [CrossRef]
- Otsuki, S.; Adachi, K.; Taguchi, T. A novel fiber-optic gas-sensing configuration using extremely curved optical fibers and an attempt for optical humidity detection. Sens. Actuators B Chem. 1998, 53, 91–96. [Google Scholar] [CrossRef]
- Jindal, R. High dynamic range fiber optic relative humidity sensor. Opt. Eng. 2002, 41, 1093. [Google Scholar] [CrossRef]
- Xu, L.; Fanguy, J.C.; Soni, K.; Tao, S. Optical fiber humidity sensor based on evanescent-wave scattering. Opt. Lett. 2004, 29, 1191. [Google Scholar] [CrossRef] [Green Version]
- Zamarreño, C.R.; Hernaez, M.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Tunable humidity sensor based on ITO-coated optical fiber. Sens. Actuators B Chem. 2010, 146, 414–417. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Hernaez, M.; Sanchez, P.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Optical fiber humidity sensor based on lossy mode resonances supported by TiO2/PSS coatings. Procedia Eng. 2011, 25, 1385–1388. [Google Scholar] [CrossRef]
- Hernaez, M.; Zamarreño, C.R.; Fernandez-Valdivielso, C.; Del Villar, I.; Arregui, F.J.; Matias, I.R. Agarose optical fibre humidity sensor based on electromagnetic resonance in the infra-red region. Phys. Status Solidi Curr. Top. Solid State Phys. 2010, 7, 2767–2769. [Google Scholar] [CrossRef]
- Sanchez, P.; Zamarreño, C.R.; Hernaez, M.; del Villar, I.; Matias, I.R.; Arregui, F.J. Humidity sensor fabricated by deposition of SnO 2 layers onto optical fibers. Fifth Eur. Work. Opt. Fibre Sens. 2013, 8794, 87940C. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Hernaez, M.; Del Villar, I.; Matias, I.R.; Arregui, F.J. Lossy mode resonance-based optical fiber humidity sensor. Proc. IEEE Sens. 2011, 2011, 234–237. [Google Scholar] [CrossRef]
- Hernaez, M.; Mayes, A.G.; Melendi-Espina, S. Lossy Mode Resonance Generation by Graphene Oxide Coatings Onto Cladding-Removed Multimode Optical Fiber. IEEE Sens. J. 2019, 19, 6187–6192. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Chen, C.; Xia, K.; Peng, S.; Guan, H.; Tang, J.; Lu, H.; Yu, J.; Zhang, J.; Xiao, Y.; et al. Tungsten disulfide (WS_2) based all-fiber-optic humidity sensor. Opt. Express 2016, 24, 8956. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Optical fiber humidity sensors based on Localized Surface Plasmon Resonance (LSPR) and Lossy-mode resonance (LMR) in overlays loaded with silver nanoparticles. Sens. Actuators B Chem. 2012, 173, 244–249. [Google Scholar] [CrossRef]
- Sanchez, P.; Zamarrẽo, C.R.; Hernaez, M.; Del Villar, I.; Fernandez-Valdivielso, C.; Matias, I.R.; Arregui, F.J. Lossy mode resonances toward the fabrication of optical fiber humidity sensors. Meas. Sci. Technol. 2012, 23, 014002. [Google Scholar] [CrossRef]
- Ascorbe, J.; Corres, J.M.; Matias, I.R.; Arregui, F.J. High sensitivity humidity sensor based on cladding-etched optical fiber and lossy mode resonances. Sens. Actuators B Chem. 2016, 233, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, O.; Corres, J.M.; Matias, I.R.; Villar, I. Del Generation of Lossy Mode Resonances in Planar Waveguides Toward Development of Humidity Sensors. J. Light. Technol. 2019, 37, 2300–2306. [Google Scholar] [CrossRef]
- Urrutia, A.; Goicoechea, J.; Rivero, P.J.; Pildain, A.; Arregui, F.J. Optical fiber sensors based on gold nanorods embedded in polymeric thin films. Sens. Actuators B Chem. 2018, 255, 2105–2112. [Google Scholar] [CrossRef]
- Bohorquez, D.L.; Del Villar, I.; Corres, J.M.; Matias, I.R. Generation of lossy mode resonances in a broadband range with multilayer coated coverslips optimized for humidity sensing. Sens. Actuators B Chem. 2020, 325, 128795. [Google Scholar] [CrossRef]
- Szulczyński, B.; Gębicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Mariammal, R.N.; Ramachandran, K.; Renganathan, B.; Sastikumar, D. On the enhancement of ethanol sensing by CuO modified SnO2 nanoparticles using fiber-optic sensor. Sens. Actuators B Chem. 2012, 169, 199–207. [Google Scholar] [CrossRef]
- Mohandoss, R.; Renganathan, B.; Annalakshmi, O.; Ganesan, A.R. Gamma radiation impact on the fiber optic acetone gas sensing behaviour of magnesium tetraborate. Opt. Fiber Technol. 2019, 52, 101935. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Taverna, G.; Bellini, A.; Eusebio, L.; Buffi, N.; Lazzeri, M.; Guazzoni, G.; Bozzini, G.; Seveso, M.; Mandressi, A.; et al. Application and uses of electronic noses for clinical diagnosis on urine samples: A review. Sensors 2016, 16, 1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhou, L.; Huang, J.; Tao, C.; Li, X.; Chen, W. Sensitivity enhancing of transition mode long-period fiber grating as methane sensor using high refractive index polycarbonate/cryptophane A overlay deposition. Sens. Actuators B Chem. 2015, 207, 477–480. [Google Scholar] [CrossRef]
- Esposito, F.; Zotti, A.; Ranjan, R.; Zuppolini, S.; Borriello, A.; Campopiano, S.; Zarrelli, M.; Iadicicco, A. Single-Ended Long Period Fiber Grating Coated with Polystyrene Thin Film for Butane Gas Sensing. J. Light. Technol. 2018, 36, 825–832. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vadivel, S. Fiber optic ethanol gas sensor based WO3 and WO3/gC3N4 nanocomposites by a novel microwave technique. Opt. Laser Technol. 2019, 118, 44–51. [Google Scholar] [CrossRef]
- Elosua, C.; Arregui, F.J.; Zamarreño, C.R.; Bariain, C.; Luquin, A.; Laguna, M.; Matias, I.R. Volatile organic compounds optical fiber sensor based on lossy mode resonances. Sens. Actuators B Chem. 2012, 173, 523–529. [Google Scholar] [CrossRef]
- Wang, T.; Okuda, H.; Lee, S.-W. Methanol selective fibre-optic gas sensor with a nanoporous thin film of organic-inorganic hybrid multilayers. Fifth Asia Pacific Opt. Sens. Conf. 2015, 9655, 96550Z. [Google Scholar] [CrossRef]
- Charles, J.; Muthusamy, S. Facile synthesis of ternary polypyrrole/Prussian blue/Titanium dioxide composite and their performance for isopropyl alcohol sensing at room temperature. AIP Conf. Proc. 2019, 2166, 020004. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Alex, Z.C. ZnO nanorods based fiber optic hexane sensor. AIP Conf. Proc. 2019, 2162, 020105. [Google Scholar] [CrossRef]
- Vadivel, S.; Balaji, G.; Rathinavel, S. High performance ethanol and acetone gas sensor based nanocrystalline MnCo2O4 using clad-modified fiber optic gas sensor. Opt. Mater. 2018, 85, 267–274. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Alex, Z.C. Fiber-optic ammonia sensor based on amine functionalized ZnO nanoflakes. IEEE Sens. J. 2018, 18, 201–208. [Google Scholar] [CrossRef]
- Subashini, T.; Renganathan, B.; Stephen, A.; Prakash, T. Acetone sensing behaviour of optical fiber clad-modified with γ-CuBr nanocrystals. Mater. Sci. Semicond. Process. 2018, 88, 181–185. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Meher, S.R.; Sivacoumar, R.; Alex, Z.C. Influence of surface functionalization on the gas sensing characteristics of ZnO nanorhombuses. J. Alloys Compd. 2017, 706, 186–197. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Meher, S.R.; Sivacoumar, R.; Alex, Z.C. ZnO nanoparticles based fiber optic gas sensor. AIP Conf. Proc. 2016, 1731, 10–13. [Google Scholar] [CrossRef]
- Renganathan, B.; Ganesan, A.R. Influence of annealing on optical fiber gas sensing properties of TiO2 nanomaterial. Int. J. ChemTech Res. 2015, 7, 878–883. [Google Scholar]
- Sharifpour-Boushehri, S.; Hosseini-Golgoo, S.M.; Sheikhi, M.H. A low cost and reliable fiber optic ethanol sensor based on nano-sized SnO2. Opt. Fiber Technol. 2015, 24, 93–99. [Google Scholar] [CrossRef]
- Devi, P.I.; Is, N.R.; Renganathan, B.; Sastikumar, D.; Ramachandran, K. Ethanol Gas Sensing of Mn-Doped CoFe204 Nanoparticles. IEEE Sens. J. 2014, 50, 3–4. [Google Scholar] [CrossRef]
- Sastikumar, D.; Renganathan, B. Sensing characteristics of clad-modified with nanocrystalline metal oxide fiber optic gas sensor. Adv. Sens. Syst. Appl. 2014, 9274, 92740S. [Google Scholar] [CrossRef]
- Noel, J.L.; Udayabhaskar, R.; Renganathan, B.; Muthu Mariappan, S.; Sastikumar, D.; Karthikeyan, B. Spectroscopic and fiber optic ethanol sensing properties Gd doped ZnO nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 634–638. [Google Scholar] [CrossRef]
- Elosúa, C.; Vidondo, I.; Arregui, F.J.; Bariain, C.; Luquin, A.; Laguna, M.; Matías, I.R. Lossy mode resonance optical fiber sensor to detect organic vapors. Sens. Actuators B Chem. 2013, 187, 65–71. [Google Scholar] [CrossRef]
- Mohandoss, R.; Dhanuskodi, S.; Renganathan, B.; Sastikumar, D. Gas sensing property of lithium tetraborate clad modified fiber optic sensor. Curr. Appl. Phys. 2013, 13, 957–963. [Google Scholar] [CrossRef]
- Mariammal, R.N.; Stella, C.; Ramachandran, K. Chunk-shaped ZnO nanoparticles for ethanol sensing. AIP Conf. Proc. 2013, 1512, 368–369. [Google Scholar] [CrossRef]
- Renganathan, B.; Ganesan, A.R. Fiber optic gas sensor with nanocrystalline ZnO. Opt. Fiber Technol. 2014, 20, 48–52. [Google Scholar] [CrossRef]
- Chou, C.H.; World Health Organization. Hydrogen Sulfide: Human Health Aspects; World Health Organization: Geneva, Switzerland, 2003; ISBN 9241530537. [Google Scholar]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2020, 27, 137–153. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef]
- Usha, S.P.; Mishra, S.K.; Gupta, B.D. Zinc oxide thin film/nanorods based lossy mode resonance hydrogen sulphide gas sensor. Mater. Res. Express 2015, 2, 95003. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors-A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Mishra, S.K.; Usha, S.P.; Gupta, B.D. A lossy mode resonance-based fiber optic hydrogen gas sensor for room temperature using coatings of ITO thin film and nanoparticles. Meas. Sci. Technol. 2016, 27. [Google Scholar] [CrossRef]
- Saidin, N.; Idris, N.F.; Yaacob, M.H.; Harun, S.W.; Md Ralib, A.A.; Hasbullah, N.F. Single-mode fiber coated with zinc oxide (ZnO) nanorods for H2 gas sensor applications. In Proceedings of the 2019 IEEE International Conference on Sensors and Nanotechnology, Penang, Malaysia, 24–25 July 2019. [Google Scholar]
- Yan, Q.; Tao, S.; Toghiani, H. Optical fiber evanescent wave absorption spectrometry of nanocrystalline tin oxide thin films for selective hydrogen sensing in high temperature gas samples. Talanta 2009, 77, 953–961. [Google Scholar] [CrossRef]
- Villar, I.; Bohorquez, D.L.; Caputo, D.; Buzzin, A.; Baldini, F.; Zamarreño, C.R.; Member, S.; Ignacio, R.; Member, S. Lossy Mode Resonance Sensors based on Tungsten Oxide Thin Films. In Proceedings of the 2020 IEEE SENSORS, Rotterdam, The Netherlands, 25–28 October 2020. [Google Scholar]
- Theoderaj, A.K.C.; Inbaraj, D.J.; Mangalaraj, C. CdS coated clad-modified fiber optic sensor for detection of NO2 gas. Mater. Res. Express 2019, 6, 1050c8. [Google Scholar] [CrossRef]
- Di Franco, C.; Elia, A.; Spagnolo, V.; Scamarcio, G.; Lugarà, P.M.; Ieva, E.; Cioffi, N.; Torsi, L.; Bruno, G.; Losurdo, M.; et al. Optical and electronic NOx sensors for applications in mechatronics. Sensors 2009, 9, 3337–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Ruan, Y.; Li, T.; Huang, J.; Warren-Smith, S.C.; Ebendorff-Heidepriem, H.; Monro, T.M. Nitric oxide optical fiber sensor based on exposed core fibers and CdTe/CdS quantum dots. Sens. Actuators B Chem. 2018, 273, 9–17. [Google Scholar] [CrossRef]
- Niyat, F.Y.; Abadi, M.H.S. the Review of Semiconductor Gas Sensor for Nox Detcting. Turkish Online J. Des. Art Commun. 2016, 6, 898–937. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NO x sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar] [CrossRef]
- Sangeetha, M.; Madhan, D. Ultra sensitive molybdenum disulfide (MoS2)/graphene based hybrid sensor for the detection of NO2 and formaldehyde gases by fiber optic clad modified method. Opt. Laser Technol. 2020, 127, 106193. [Google Scholar] [CrossRef]
- John, M.S.; Thomas, J.; Unnikrishnan, K.P.; Radhakrishnan, P. NO2 detection with a fibre optic evanescent wave sensor. SPIE Conf. Adv. Photonic Sens. Appl. 1999, 3897, 173–178. [Google Scholar]
- Fuentes, O.; Goicoechea, J.; Corres, J.M.; Del Villar, I.; Ozcariz, A.; Matias, I.R. Generation of lossy mode resonances with different nanocoatings deposited on coverslips. Opt. Express 2020, 28, 288. [Google Scholar] [CrossRef]
- Astrain, J.J.; Falcone, F.; Lopez, A.; Sanchis, P.; Matias, J.V.I.R. Monitoring of Electric Buses within an Urban Smart City Environment. In Proceedings of the IEEE Sensors, Rotterdam, The Netherlands, 25–28 October 2020; pp. 1–4. [Google Scholar]





| Detected Gas | Material | Deposition Technique | Gas Concentration | Sensitivity | Optical Waveguide | Detection Limit | Reference |
|---|---|---|---|---|---|---|---|
| H2S | (a) ZnO thin-film Zno nanoparticles, (b) Zno nanoparticles (c) SPR (silver +ZnO) | Thermal evaporation technique and nanoparticles dipping | 10 to 100 ppm | (a) 1.49, (b) 1.06 and (c) 0.24 nm ppm−1 | PCS 600 µm | 10 ppm * | [30] |
| H2S | (a) ZnO nanorods | Hydrothermal growth method and thermal evaporation coating | 10 to 100 ppm | (a) 4.14 nm ppm−1 at 10 ppm | PCS 600 µm | 10 ppm * | [119] |
| (b) ZnO film and nanorods | (b) 2.35 nm ppm−1 film at 10 ppm | ||||||
| H2 | WO3 y Pt | Dip coating | 1 vol.% H2–99 vol.% N2 | N/A | 200 µm micron quartz-core/plastic-cladding | 1 vol.% H2–99 vol.% N2 * | [21] |
| H2 | nanocrystalline SnO2 | Dip coating | 0.1 to 10% H2 | N/A | gold-jacketed optical fiber | 0.1% * | [123] |
| H2 | (a) ITO film and nanoparticles (b) ITO nanoparticles (c) ITO film | Thermal evaporation technique, dip coating for nanoparticles | 0 ppm to 100 ppm | (a) 0.71 (b) 0.58 (c) 0.2 nm ppm−1 | NA 0.37 600 µm | 10 ppm * | [121] |
| H2 | ZnO nanorods | Growth solution | 0.25% | N/A | SMF fiber | 0.25% * | [122] |
| NO | CdTe/CdS quantum dots | Sinalization | 10–11 to 10–4 mol/L | 0.3412 I/log[(NO)] | Exposed core fiber fabricated in-house | 10 pmol/L | [127] |
| NO2 | Metallophthalocyanine (MPc) | Thermally deposited | N/A | N/A | Multimode 200 µm | N/A | [131] |
| NO2 | CdS | Chemical Bath Deposition (CBD) | 0 to 700 ppm | 63.4% at 600 ppm | PMMA 750 μm | 100 ppm * | [125] |
| NO2 | Molybdenum disulfide (MoS2)/graphene | Chemical method | 0 to 500 ppm | 61% | PMMA 650 µm | 100 ppm * | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitoria, I.; Ruiz Zamarreño, C.; Ozcariz, A.; Matias, I.R. Fiber Optic Gas Sensors Based on Lossy Mode Resonances and Sensing Materials Used Therefor: A Comprehensive Review. Sensors 2021, 21, 731. https://0-doi-org.brum.beds.ac.uk/10.3390/s21030731
Vitoria I, Ruiz Zamarreño C, Ozcariz A, Matias IR. Fiber Optic Gas Sensors Based on Lossy Mode Resonances and Sensing Materials Used Therefor: A Comprehensive Review. Sensors. 2021; 21(3):731. https://0-doi-org.brum.beds.ac.uk/10.3390/s21030731
Chicago/Turabian StyleVitoria, Ignacio, Carlos Ruiz Zamarreño, Aritz Ozcariz, and Ignacio R. Matias. 2021. "Fiber Optic Gas Sensors Based on Lossy Mode Resonances and Sensing Materials Used Therefor: A Comprehensive Review" Sensors 21, no. 3: 731. https://0-doi-org.brum.beds.ac.uk/10.3390/s21030731