Association between Sleep Quality and Body Composition in Sedentary Middle-Aged Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sleep Quality Assessment
2.3. Anthropometric and Body Composition Assessment
2.4. Statistical Analysis
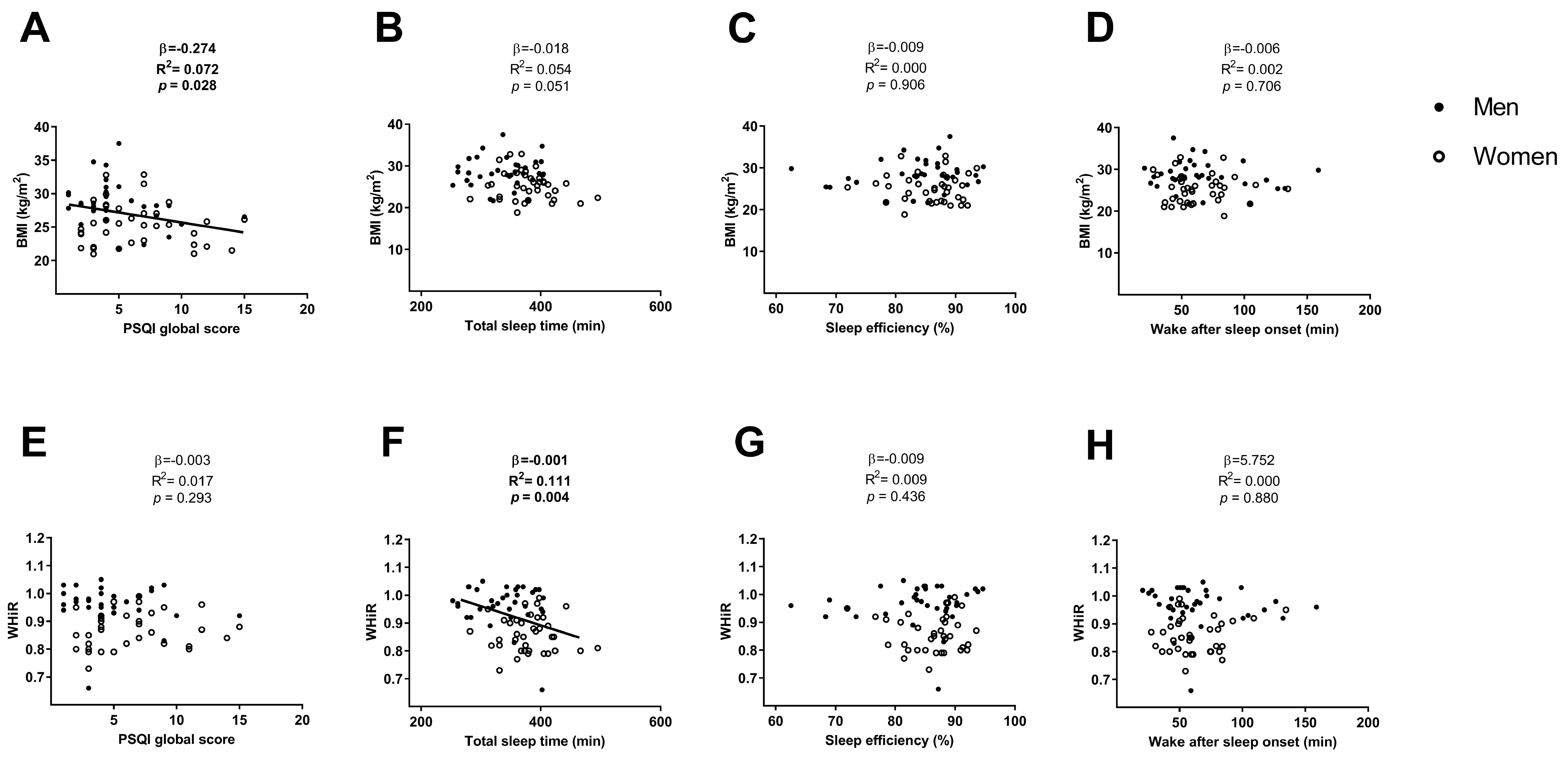
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohara, K. Sarcopenic obesity in aging population: Current status and future directions for research. Endocrine 2014, 45, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.A.; Heymsfield, S.B. Implications of low muscle mass across the continuum of care: a narrative review. Ann. Med. 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; O’Donovan, G. Sarcopenic obesity, weight loss, and mortality: The English Longitudinal Study of Ageing. Am. J. Clin. Nutr. 2017, 106, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gadie, A.; Shafto, M.; Leng, Y.; Kievit, R.A. How are age-related differences in sleep quality associated with health outcomes ? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 2017, 7, e014920. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011, 21, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao, X.Q.; Liu, X.; Deng, H.; Chan, T.; Ho, K.F.; Wang, F.; Vermeulen, R.; Tam, T.; Wong, M.C.; Tse, L.A.; et al. Sleep quality, sleep duration, and the risk of coronary heart disease: A prospective cohort study with 60, 586 adults. J. Clin. Sleep Med. 2018, 14, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Chen, P.; Zhang, L.; Zhang, P.; Yu, J.; Zhang, N. Relation of sleep quality and sleep duration to type 2 diabetes: A population-based cross-sectional survey. BMJ 2012, 2, e000956. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Scheer, F.A. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leary, K.O.; Bylsma, L.M.; Rottenberg, J.; Leary, K.O.; Bylsma, L.M.; Why, J.R. Why might poor sleep quality lead to depression? A role for emotion regulation regulation. Cogn. Emot. 2016, 31, 1698–1706. [Google Scholar] [CrossRef]
- Albayrak, I.; Aydogmus, M.; Ozerbil, O.M.; Levendoglu, F. The association between bone mineral density, quality of life, quality of sleep and fatigue. Acta Clin. Belg. 2016, 71, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, N.; Spira, D.; Norman, K.; Demuth, I.; Eckardt, R.; Steinhagen-Thiessen, E. Sleep, muscle mass and muscle function in older people: a cross-sectional analysis based on data from the Berlin Aging Study II (BASE-II). Dtsch. Arztebl. Int. 2016, 113, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Karschin, J.; Breusing, N.; Bosy-Westphal, A.; Kahlh, J. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity 2016, 24, 335–341. [Google Scholar] [CrossRef]
- Sasaki, N.; Fujiwara, S.; Yamashita, H.; Ozono, R.; Teramen, K.; Kihara, Y. Impact of sleep on osteoporosis: Sleep quality is associated with bone stiffness index. Sleep Med. 2016, 25, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.D.; Abucham, J.; dos Santos, R.V.T.; Mello, M.T.; Tufik, S.; Poyares, D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res. Rev. 2015, 23, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Beccuti, G.; Pannain, S. Sleep and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Espuch-Oliver, A.; Robles-Gonzalez, L.; Navarro-Lomas, G.; de Haro, T.; Femia, P.; Castillo, M.J.; Gutierrez, A. Exercise training as S-Klotho protein stimulator in sedentary healthy adults: Rationale, design, and methodology. Contemp. Clin. Trials Commun. 2018, 11, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Migueles, J.H.; Ulf, C.C.; Nystro, C.D. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. Sport. Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, D.; Jung, S.; Saadat, M.; Sirohi, R.; Crewson, K. How to interpret the results of a sleep study. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 24983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OMS | Obesidad y Sobrepeso. WHO 2016. Available online: http://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 November 2018).
- Lucassen, E.A.; de Mutsert, R.; le Cessie, S.; Appelman-Dijkstra, N.M.; Rosendaal, F.R.; van Heemst, D.; den Heijer, M.; Biermasz, N.R.; NEO Study Group. Poor sleep quality and later sleep timing are risk factors for osteopenia and sarcopenia in middle-aged men and women: The NEO study. PLoS ONE 2017, 12, e0176685. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, E.A.; Coomans, C.P.; van Putten, M.; de Kreij, S.R.; van Genugten, J.H.; Sutorius, R.P.; de Rooij, K.E.; van der Velde, M.; Verhoeve, S.L.; Smit, J.W.; Löwik, C.W. Environmental 24-hr cycles are essential for health. Curr. Biol. 2016, 26, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar] [PubMed]
- Lacativa, P.G.S.; Farias, M.L.F.D. Osteoporosis and inflammation. Arq. Bras. Endocrinol. Metab. 2010, 54, 123–132. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, F.; He, H. Melatonin effects on hard tissues: Bone and tooth. Int. J. Mol. Sci. 2013, 14, 10063–10074. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Scillitani, A. Role of cortisol hypersecretion in the pathogenesis of osteoporosis. Recenti Prog. Med. 2008, 99, 309–313. [Google Scholar] [PubMed]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2016, 64, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, E.; El, R.H.; Zunquin, G.; Jacob, C.; Moussa, E.; Theunynck, D. Sleep quality is a determinant of hip bone mineral density in a group of young Lebanese men. J. Med. Liban. 2014, 62, 213–216. [Google Scholar] [PubMed]
- Saint Martin, M.; Labeix, P.; Garet, M.; Thierry, T.; Barthélémy, J.C.; Collet, P.; Sforza, E. Does subjective sleep affect bone mineral density in older people with minimal health disorders? The PROOF cohort. J. Clin. Sleep Med. 2016, 12, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Goodin, B.R.; Smith, M.T.; Quinn, N.B.; King, C.D.; McGuire, L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol. Psychol. 2012, 91, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, G.M.E.E.; Van Schoor, N.M.; Van Rossum, E.F.C.; Visser, M.; Lips, P.T.A.M. The relationship between cortisol, muscle mass and muscle strength in older persons and the role of genetic variations in the glucocorticoid receptor. Clin. Endocrinol. (Oxf.) 2008, 69, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Rusch, H.L.; Guardado, P.; Baxter, T.; Mysliwiec, V.; Gill, J.M. Improved sleep quality is associated with reductions in depression and PTSD arousal symptoms and increases in IGF-1 concentrations. J. Clin. Sleep Med. 2015, 11, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Pyykkönen, A.J.; Isomaa, B.; Pesonen, A.K.; Eriksson, J.G.; Groop, L.; Tuomi, T.; Räikkönen, K. Subjective sleep complaints are associated with insulin resistance in individuals without diabetes. Diabetes Care 2012, 35, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Biol. 2013, 45, 2147–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J. Obesity in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal. Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.S.; Banks, S.; Arroyo, S.; Dinges, D.F. Effects of sleep restriction on adiponectin levels in healthy men and women. Physiol. Behav. 2010, 101, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goropashnaya, A.V.; Herron, J.; Sexton, M.; Havel, P.J.; Stanhope, K.L.; Plaetke, R.; Mohatt, G.V.; Boyer, B.B. Relationships between plasma adiponectin and body fat distribution, insulin sensitivity, and plasma lipoproteins in Alaskan Yup’ik eskimos: The CANHR study. Metabolism 2009, 58, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Abate, N. Body fat distribution and insulin resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Rahe, C.; Czira, M.E.; Teismann, H.; Berger, K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015, 16, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, R.; Marventano, S.; Castellano, S.; Giogianni, G.; Nolfo, F.; Rametta, S.; Matalone, M.; Mistretta, A. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Sci. 2016, 9, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mallampalli, M.P.; Carter, C.L. Exploring sex and gender differences in sleep health: A society for women’s health research report. J. Womens Health (Larchmt) 2014, 23, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Collop, N.A. Gender differences in sleep disorders. Curr. Opin. Pulm. Med. 2006, 12, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Santhi, N.; Lazar, A.S.; Mccabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, I.; Obeid, J.; Timmons, B.W.; DeMatteo, C. Exploring accelerometer versus self-report sleep assessment in youth with concussion. Glob. Pediatr. Health 2017, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]



| All (n = 74) | Men (n = 35) | Women (n = 39) | |
|---|---|---|---|
| Age (years) | 53.7 ± 5.1 | 54.4 ± 5.3 | 53.0 ± 5.0 |
| Body composition parameters | |||
| Body mass index (kg/m2) | 26.7 ± 3.8 | 28.3 ± 3.6 | 25.3 ± 3.3 |
| Waist-Hip ratio | 0.91 ± 0.08 | 0.97 ± 0.07 | 0.86 ± 0.06 |
| Bone mineral content (g) | 2258.1 ± 453.5 | 2633.6 ± 301.2 | 1921.1 ± 259.8 |
| Bone mineral density (g/cm2) | 1.10 ± 0.10 | 1.16 ± 0.08 | 1.05 ± 0.09 |
| Lean mass (kg) | 43.5 ± 11.7 | 53.9 ± 6.5 | 34.1 ± 5.8 |
| Lean mass index (kg/m2) | 15.2 ± 2.9 | 17.5 ± 2.0 | 13.2 ± 1.8 |
| Fat mass (kg) | 30.0 ± 8.4 | 30.9 ± 9.8 | 29.2 ± 7.1 |
| Fat mass (%) | 39.9 ± 9.1 | 34.7 ± 8.0 | 44.5 ± 7.4 |
| Fat mass index (kg/m2) | 10.7 ± 3.1 | 10.0 ± 3.2 | 11.4 ± 2.9 |
| Sleep quality parameters | |||
| PSQI global score | 5.6 ± 3.5 | 4.8 ± 3.1 | 6.3 ± 3.6 |
| Total sleep time (min) | 359.9 ± 48.8 | 337.9 ± 46.3 | 380.1 ± 42.4 |
| Sleep efficiency (%) | 85.0 ± 6.3 | 84.9 ± 7.5 | 86.1 ± 4.7 |
| Wake after sleep onset (min) | 63.9 ± 27.4 | 65.8 ± 32.4 | 62.2 ± 22.2 |
| All (n = 74) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Body Composition Parameters | β | R2 | p | β | R2 | p | β | R2 | p |
| Body mass index (kg/m2) | −0.188 | 0.204 | 0.112 | −0.241 | 0.094 | 0.057 | −0.144 | 0.127 | 0.279 |
| Waist-Hip ratio | −5.498 | 0.345 | 0.982 | −0.004 | 0.050 | 0.178 | −1.030 | 0.111 | 0.867 |
| Bone mineral content (g) | −20.624 | 0.634 | 0.045 | −42.544 | 0.107 | 0.009 | −0.002 | 0.144 | 0.121 |
| Bone mineral density (g/cm2) | −0.010 | 0.419 | 0.001 | −0.013 | 0.197 | <0.001 | −14.293 | 0.230 | 0.003 |
| Lean mass (kg) | −0.466 | 0.725 | 0.044 | −1.001 | 0.115 | 0.016 | −8.629 | 0.129 | 0.259 |
| Lean mass index (kg/m2) | −0.238 | 0.640 | <0.001 | −0.332 | 0.228 | 0.001 | −0.724 | 0.219 | 0.004 |
| Fat mass (kg) | 0.331 | 0.026 | 0.255 | 0.274 | 0.014 | 0.346 | 5.945 | 0.129 | 0.256 |
| Fat mass (%) | 0.511 | 0.353 | 0.058 | 0.765 | 0.106 | 0.016 | 0.083 | 0.140 | 0.150 |
| Fat mass index (kg/m2) | 0.072 | 0.068 | 0.506 | 0.116 | 0.018 | 0.297 | 0.077 | 0.115 | 0.588 |
| Visceral adipose tissue (g) | 6.407 | 0.188 | 0.591 | −5.293 | 0.006 | 0.687 | 0.001 | 0.115 | 0.583 |
| All (n = 74) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Body Composition Parameters | β | R2 | p | β | R2 | p | β | R2 | p |
| Body mass index (kg/m2) | −0.005 | 0.182 | 0.622 | −0.019 | 0.083 | 0.040 | −0.876 | 0.192 | 0.587 |
| Waist-Hip ratio | 0.000 | 0.391 | 0.455 | −0.001 | 0.126 | 0.006 | −58.250 | 0.195 | 0.467 |
| Bone mineral content (g) | −0.441 | 0.639 | 0.565 | −3.664 | 0.152 | 0.001 | −0.012 | 0.193 | 0.532 |
| Bone mineral density (g/cm2) | 0.000 | 0.352 | 0.120 | −0.001 | 0.159 | 0.001 | −102.173 | 0.219 | 0.110 |
| Lean mass (kg) | −0.004 | 0.734 | 0.825 | −0.096 | 0.186 | 0.001 | 0.000 | 0.190 | 0.718 |
| Lean mass index (kg/m2) | −0.003 | 0.563 | 0.544 | −0.023 | 0.237 | <0.001 | −2.916 | 0.198 | 0.393 |
| Fat mass (kg) | 0.007 | 0.019 | 0.775 | −0.003 | 0.010 | 0.870 | 0.000 | 0.190 | 0.765 |
| Fat mass (%) | 0.006 | 0.275 | 0.774 | 0.049 | 0.109 | 0.022 | 0.261 | 0.190 | 0.724 |
| Fat mass index (kg/m2) | −7.391 | 0.039 | 0.993 | 0.006 | 0.016 | 0.451 | 0.020 | 0.189 | 0.991 |
| Visceral adipose tissue (g) | −0.576 | 0.221 | 0.548 | −2.049 | 0.073 | 0.033 | −0.009 | 0.193 | 0.555 |
| All (n = 74) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Body Composition Parameters | β | R2 | p | β | R2 | p | β | R2 | p |
| Body mass index (kg/m2) | 3.686 | 0.183 | 0.584 | −1.190 | 0.024 | 0.869 | 0.120 | 0.035 | 0.598 |
| Waist-Hip ratio | 0.020 | 0.386 | 0.879 | −0.122 | 0.028 | 0.460 | 1.861 | 0.031 | 0.869 |
| Bone mineral content (g) | 71.598 | 0.637 | 0.896 | −960.826 | 0.019 | 0.279 | 0.000 | 0.031 | 0.918 |
| Bone mineral density (g/cm2) | −0.101 | 0.332 | 0.545 | −0.266 | 0.031 | 0.180 | −5.737 | 0.036 | 0.527 |
| Lean mass (kg) | −2.926 | 0.734 | 0.807 | −32.049 | 0.057 | 0.152 | −0.053 | 0.033 | 0.716 |
| Lean mass index (kg/m2) | −2.532 | 0.564 | 0.502 | −8.966 | 0.123 | 0.092 | −0.435 | 0.043 | 0.365 |
| Fat mass (kg) | 23.375 | 0.047 | 0.150 | 20.077 | 0.032 | 0.212 | 0.132 | 0.061 | 0.148 |
| Fat mass (%) | 15.583 | 0.286 | 0.298 | 29.174 | 0.079 | 0.044 | 0.116 | 0.049 | 0.261 |
| Fat mass index (kg/m2) | 6.054 | 0.053 | 0.313 | 7.752 | 0.032 | 0.196 | 0.254 | 0.046 | 0.306 |
| Visceral adipose tissue (g) | 148.816 | 0.217 | 0.827 | −342.253 | 0.011 | 0.651 | 0.000 | 0.031 | 0.824 |
| All (n = 74) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Body Composition Parameters | β | R2 | p | β | R2 | p | β | R2 | p |
| Body mass index (kg/m2) | −0.010 | 0.184 | 0.502 | −0.006 | 0.025 | 0.739 | −0.634 | 0.011 | 0.528 |
| Waist-Hip ratio | −7.338 | 0.386 | 0.807 | 4.170 | 0.020 | 0.912 | −13.219 | 0.006 | 0.791 |
| Bone mineral content (g) | −0.414 | 0.637 | 0.737 | 0.509 | 0.003 | 0.803 | −0.004 | 0.007 | 0.771 |
| Bone mineral density (g/cm2) | 0.000 | 0.329 | 0.745 | 0.000 | 0.010 | 0.546 | 14.698 | 0.007 | 0.714 |
| Lean mass (kg) | 0.004 | 0.734 | 0.897 | 0.031 | 0.034 | 0.551 | 0.186 | 0.007 | 0.772 |
| Lean mass index (kg/m2) | 0.005 | 0.563 | 0.580 | 0.011 | 0.096 | 0.365 | 1.764 | 0.016 | 0.406 |
| Fat mass (kg) | −0.055 | 0.049 | 0.136 | −0.053 | 0.040 | 0.151 | −0.604 | 0.039 | 0.132 |
| Fat mass (%) | −0.034 | 0.285 | 0.321 | −0.047 | 0.023 | 0.224 | −0.506 | 0.023 | 0.269 |
| Fat mass index (kg/m2) | −0.014 | 0.054 | 0.307 | −0.016 | 0.027 | 0.253 | −1.152 | 0.022 | 0.294 |
| Visceral adipose tissue (g) | −0.510 | 0.218 | 0.741 | −0.107 | 0.008 | 0.951 | −0.003 | 0.007 | 0.735 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la-O, A.; Dote-Montero, M.; Gutiérrez, Á.; Castillo, M.J. Association between Sleep Quality and Body Composition in Sedentary Middle-Aged Adults. Medicina 2018, 54, 91. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050091
Jurado-Fasoli L, Amaro-Gahete FJ, De-la-O A, Dote-Montero M, Gutiérrez Á, Castillo MJ. Association between Sleep Quality and Body Composition in Sedentary Middle-Aged Adults. Medicina. 2018; 54(5):91. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050091
Chicago/Turabian StyleJurado-Fasoli, Lucas, Francisco J. Amaro-Gahete, Alejandro De-la-O, Manuel Dote-Montero, Ángel Gutiérrez, and Manuel J. Castillo. 2018. "Association between Sleep Quality and Body Composition in Sedentary Middle-Aged Adults" Medicina 54, no. 5: 91. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54050091





