1. Introduction
Pharmaceutical excipients for direct compression (DC) applications are mostly favored in relation to saving time, cost and labour for solid dosage form preparations and tableting [
1,
2]. The foregoing advantages are due to their ability to provide the three main requirements associated with excipients for DC processing, i.e., compressibility, compactibility, and flowability [
3,
4]. Many DC excipients are manufactured from natural sources (e.g., cellulose and starch), from existing excipients of synthetic origin or from binary mixtures of non-DC excipients [
5,
6]. The necessity for structural modification and industrial manufacture is attributed to the detrimental physical properties of most pharmaceutical excipients before being processed. These properties include poor compactibility, compressibility, and flowability.
Industrially, different processes have been used in the scale-up production of DC excipients. Spray-drying and spray-granulation represent the most two common processes in DC excipient production [
7,
8,
9]. However, apart from the high cost and investment of time, these techniques commonly impose complexity in terms of operational procedures, as well as process control [
10]. Moreover, prior to spray drying, most excipients are subjected to physical and chemical treatment in order to provide specific functionalities for in vivo drug delivery purposes [
11,
12]. Such pre-treatment steps add to the complexity of product manufacture.
Dry granulation represents a preferred industrial alternative in order to minimize time and cost for a myriad of pharmaceutical applications. This is due to the fact that neither liquids nor heat is involved in the dry processing of powders. Arguably, the most promising dry granulation technique, to date, is roller compaction, since it has proved to be effective in replacing powders that are conventionally processed using wet granulation [
13,
14]. However, most applications of roller compactors are confined to the improvement of powder flow of pharmaceutical preparations comprising mixtures of API(s) and excipient(s) [
15]. Nevertheless, there have been attempts to employ roller compaction technology for the conversion of poorly compressible/compactable starch and α-lactose monohydrate into DC excipients [
16,
17]. In this regard, specific intensive compaction pressures were able to produce DC excipients via the mechanism of gelatinization and reduction in crystallinity for starch and α-lactose monohydrate, respectively.
Recently, there has been a significant interest in the development of chitin for pharmaceutical use, especially in direct compression processing. The basic asset of chitin that renders such a development to be advantageous lies in its ability to provide vital multi-functionalities in tablet processing. In this regard, chitin showed good tabletability, fast disintegration properties, in addition to improved flowability, compressibility, and compactibility when processed with other common excipients, such as calcium carbonate and magnesium silicate [
18,
19,
20,
21,
22,
23]. Despite the foregoing comments, chitin lacks essential manufacturing requirements for the processing of DC excipients. In this regard, the low bulk density and poor powder flowability represent the two major inherent shortcomings of chitin. Nevertheless, numerous attempts have been made to convert chitin into a pharmaceutical DC excipient. Most of these attempts have adopted co-processing techniques, whereby another excipient has been involved in, e.g., wet granulation methodologies for product manufacturing [
20,
23]. However, the manufacturing procedures and processing time and cost of such methodologies are, relatively, complex. This necessitates searching for new technical alternatives for the processing of chitin in order for it to be used as an excipient with DC functionality.
The research reported herein attempts to extend the usefulness and opportunities that roller compaction may provide in obtaining a new DC excipient using chitin. Because roller compaction is a pressure inducing technique, it was concurrently compared with ball milling in order to further support an understanding of the performance of modified chitin, as an excipient, when subjected to pressure.
3. Discussion
The research presented herein is mainly focused on the assessment of the use of roller compaction technique for the development of DC pharmaceutical excipients from raw alpha chitin. Ball milling, as another stress-inducing technique, was tested for comparison and evaluation of changes in the physical properties and compression behavior of chitin powder. The main challenge in this work is to obtain workable chitin with suitable DC properties to facilitate compaction and compression in the industrial set up. FTIR test of ball milled, and compacted chitin indicates that no chemical change took place during these processes. This guarantees that the intended excipients are chemically stable when exposed to ball milling or compaction. Another objective was to reduce crystallinity to facilitate flow and compression. In this regard, materials are known to undergo plastic deformation with regular particle shape and consequently improved flowability once their amorphous character is increased [
30]. Testing the raw chitin showed the necessity to alter the physical state of chitin, namely particle size and crystallinity. In this work, XRPD was used to assure that crystallinity raw chitin was modified, enabling treated chitin to be utilized in pharmaceutical processing.
XRPD results indicated that roller compaction and ball milling techniques affect the crystalline structure of chitin in different ways. A decrease in crystallinity of the semi-crystalline structure of raw chitin was generally the predominant change in the crystal lattice enhanced by both techniques (
Figure 2). However, this decrease is more pronounced by ball milling when operated for 36 h. In fact, ball milling converts chitin into a material of a highly amorphous character. In this regard, the decrease in the two main crystalline planes of α-chitin represented by (010) and (020) at 2θ = 9° and 19°, respectively indicates a change in the crystalline nature of chitin. Ioelovich [
31] suggests the use of integral intensities (areas) of the X-diffractions, especially for the (020) plane, instead of peak height as an indication of crystalline and amorphous contents for chitin. Based upon his finding, irrespective of which plane is more indicative of any crystallinity change, the summation of the peak areas of the two planes (010) and (020) follows the decreasing order: Raw, compacted, and ball milled. Such crystallinity change caused by roller compaction on chitin is similar to reported results on the effect of roller compaction on α-lactose monohydrate [
17]. On the other hand, Alves et al. showed that ball milling of chitosan (de-acetylated chitin) caused a loss of the crystal plane (010); however, there was an increase in the intensity of the peak at the 020 plane [
32]. The latter change further contradicts the finding by Ioelovich [
31] who—as previously mentioned—states that crystallinity changes are associated with changes in the 020 crystal plane. In order to understand the foregoing contradiction, it is suggested that the dissimilarity with the findings of Alves et al., is more likely to be attributed to the fact that milling—in their work—was carried out for a maximum time of 3 h. The foregoing is too short to represent the extensive duration of the ball milling undertaken in the current work. Therefore, it is the intense stress applied to the powder that imparts a high reduction in its crystallinity. In fact, high stress induction applied to a structurally similar polysaccharide powder, e.g., cellulose, was reported to reduce crystallinity to 54.1% when using simple crushing, and down to 21.7% upon using ball milling [
33]. Therefore, for the two techniques employed in this work, it is correct to assert that roller compaction—as a scalable process—can be regarded as a short time ball milling which, in the case of the latter method, is hard to scale-up.
At the molecular level, it has been reported that destruction of both planes at 2θ = 9° and 19° causes a reduction in the hydrogen bond network which is responsible for imparting structural integrity and flexibility to the chitin chains [
34]. Other studies (see Reference [
33]) have reported that such a reduction causes a loss in the glycosidic linkages connecting acetylated glucosamine subunits which form the main structural backbone of chitin [
33]. On the other hand, the increase in the amorphous character was further evident in the broadening and decrease in band intensities for the IR bands of ball milled chitin (
Figure 3C) when the latter is compared with raw chitin (
Figure 3A). However, changes in crystallinity for compacted chitin were not detectable using the same technique as the IR bands of roller compacted chitin did not undergo any changes (
Figure 3B).
Moving from the molecular level to the particle behavior of the chitin powder, the first most crucial property to investigate was bulk density with respect to its impact on pharmaceutical processing of powders. In this regard, increasing the bulk density is advantageous in tablet manufacturing, whereby bulky materials enhance powder compression processing, and more specifically, in die filling procedures [
35]. The increase in bulk density of chitin, due to ball milling is attributed to the size reduction of the particles, due to high energy impacts between the chitin particles during collision. In comparing the two different stress-inducing techniques, ball milling of chitin is not as good as a powder densification technique compared to roller compaction; the later methodology, imparted a greater increase in the powder bulk density.
The increase in bulk and tapped densities of chitin, due to humidification is attributed to the low optimal packing caused by strong cohesion -due to interparticle water bridging- between the molecules. Such behavior is generally anticipated when the water content of a material is increased, due to humidification as typically induced in the current case [
36]. This further suggests an improvement in particle packing when the powders were subjected to drying for four days. In this regard, dried powder presented the highest recorded bulk and tapped densities in all cases.
The second most affected factor in dry granulation is particle size distribution. Ball milling is well known as a particle size reducing technique, due to the high energy of impact when the balls collide with chitin [
37]. On the other hand, roller compaction is used for powder densification. Consequently, the two techniques are diametric to each other with respect to the changes, increasing or decreasing, they impart to particle size. Although all powders were passed and collected through sieves between 250 and 90 μm, roller compaction imparts high size distribution towards the upper limit of particle size between the aforementioned sieve ranges (
Table 3). In contrast, ball milling imparts a high distribution towards the lower limit of particle size. These changes in particle size impart another opposing explanation to the bulk density increase caused by ball milling and roller compaction. In the former, reduction of particle size renders a fixed sample volume to be occupied by small chitin particles rather than large ones. Thus, a higher bulk density is attained upon milling compared to the raw material. On the other hand, despite the increase in particle size in case of roller compaction, the extent of densification is suggested to be high enough to overcome the effect of particle size towards variations in bulk density. It has to be emphasized herein that the aforementioned particle size and bulk density of compacted chitin were only possible after the powder was allowed to be compacted five times up to an applied pressure of 166 Mpa.
The two aforementioned properties; i.e., density and size of the granules, have a direct impact on powder flow, especially when the powders are subjected to humidification. In this regard, the improved flow for raw and ball milled chitin powders subjected to high humidification conditions is suggested to be attributed to the lubrication effect generally induced by water molecules [
38,
39]. Such a lubrication-flow theory is also aligned with the apparent poor powder flow when such an effect is reduced as the materials undergo drying (
Table 4). However, the same theory does not align with the excellent powder flow when compacted chitin was dried. In this case, it is suggested that the effect of bulk density may have overcome the aforementioned concerns related to water content on powder flow. In other words, the significant increase in bulk density noticed for compacted chitin compared to the raw and ball milled chitin has rendered the compacted powders highly flowable. Drying further increases the bulk density value and bulk density difference of the compacted compared to the raw and ball milled chitin powders, and this may justify the excellent flowability of dried compacted chitin. The same justification is valid when compacted chitin was subjected to humidification. In this regard, the sharp decrease in the bulk density of the humidified compacted powder is the main reason behind its poor flowability.
Measuring changes in true density for each stress-inducing technique was tested despite the fact that, theoretically, the true density value of chitin was expected not to be affected by means of pore volume reduction. This is based on the fact that the value is constant for solid matter and excludes any empty space considerations upon measurement. Such a perspective is valid for the material subjected to roller compaction. However, it has been reported that a reduction in the true density of a material can take place when the structure undergoes a polymorphic transformation [
40,
41]. In this regard, changes in the molecular arrangement of a powder can result in loose packing at the particulate level. Generally, amorphous materials have a lower density than their crystalline counterparts as the atoms of the former are located at further distances from each other than the latter. Therefore, a decrease in crystallinity results in an increase in lattice volume, and thereby, a decrease in density [
42]. Thus, the decrease in crystallinity of chitin to a less ordered amorphous structure is suggested to be the main reason behind the decrease in true density of chitin upon ball milling. Water content, on the other hand, lowers the true density of raw chitin, since the measured value is the sum of the true densities of both water and chitin. When water is removed, the measurement will solely include the solid material and without the presence of other materials which disrupt the volume needed to be occupied by chitin for measurement [
43]. Based upon the foregoing, the true densities of dried materials, unprocessed and processed, were the highest compared to the humidified ones.
The last property to be tested, and one which varies with pressure, is a specific surface area. Initially, the high specific surface area of raw chitin is attributed to its highly porous structure [
44]. The increase in particle surface area, due to ball milling is typical behavior for a size reducing technique that principally imparts high energy upon collision of the falling balls with powder particles. In contrast, the decrease in particles surface area, due to roller compaction is more likely to be attributed to extensive folding and packing of the particles. Such densification is responsible for reducing the porous structure of raw chitin, and consequently, a decrease in the specific surface area is attained.
Although an increase in the surface area of granules is advantageous in providing new fresh particles surfaces and extra contact points for binding [
45], the crushing force of compacted powders of lower surface area was the highest. In contrast, the increase in surface area upon ball milling had no significant effect on crushing force in comparison with roller compaction. Hence, the mechanism for strong binding between the granules is not directly related to the actual surface area of the granules. In fact, it is more likely to be attributed to the mode of deformation when a force is applied. In this regard, plastic deformation of chitin enables extensive folding, thereby providing new surfaces for bridging via new contact points. On the other hand, the presence of water, when the materials were humidified, is a typical action of a granulating medium. The foregoing is known to enforce greater adhesion between wet than dried surfaces [
46]. This justifies the increase in crushing force for all the materials when they were humidified.
The hard granules produced by roller compaction showed high resistance to compression force rendering the granules with low maximum volume reduction or ‘a’ values. Concurrently, the same hard granules manifested the highest compression force (Pk) needed for (a/2) volume reduction as well as minimal particle rearrangement compared to ball milled, and raw chitin. The closer crushing force values of ball milled to raw chitin further rendered similarly closer a, PK and ab values. Accordingly, ball milled chitin particles do not provide any added value to the compressibility and compactibility of raw chitin.
When all the materials were humidified, the compressibility (represented by the ‘a’ and to some extent the ‘ab’ parameters), underwent an improvement. This is due to the fact that water acts as a plasticizer. The foregoing statement can be justified by the removal of water, as the compressibility of the dried materials decreased. On the other hand, high humidification levels (95% RH) weakened the inter-particulate bonding for all materials as the PK required for (a/2) volume reduction underwent a decrease; thus, resulting in weak humidified granules and for the decrease in tablet hardness attained when the powders were compressed.
The high compactibility of roller compacted chitin powder was further confirmed using the
PY parameter from the Heckel model. A lower
PY value is preferable for plastically deforming materials, as it indicates a higher extent of deformation. The foregoing gives rise to a greater degree of folding, and thus, the appearance of fresh new particle surfaces for bridging with nearby surfaces [
47]. Roller compacted powders manifested a high extent of plastic deformation compared to ball milled chitin which, in turn, displayed greater plasticity than the raw chitin. It is suggested that such high plasticity is attributed to the presence of denser chitin particles, due to roller compaction, and thus, new added surfaces are available for deformation. On the other hand, the high plastic deformation presented when the materials were subjected to humidification is, as stated previously, attributed to the plasticizing effect of water.
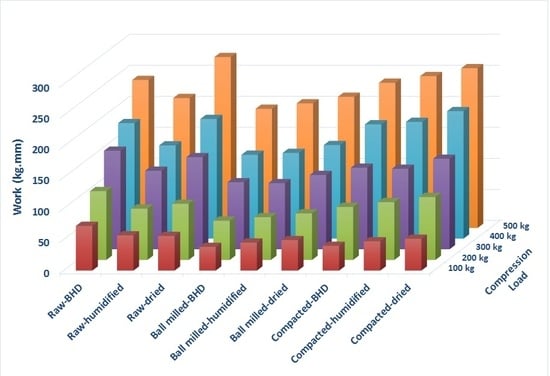
The compression force and the volume reduction can be combined together to describe the work of compression manifested by the area under the F-D curve. The fact that the work of compression is the product of force applied (f) × the displacement (d) of the compressed powder, means that this reflects how easy or hard the granules can be compressed, and/or reflects the extent of plastic/elastic or brittle deformation the particles are undergoing when pressure is applied. Because compressibility is a volume reduction parameter that has a physical displacement implication (d), it is suggested that the high Wc values for raw chitin- compared to ball milled- are attributed to its high a value. Thus, the impact of Wc was found to be valid at all compression pressures used. Similarly, using the fxd correlation to interpret Wc data, high Wc values of dried- compared to humidified- powders are more likely to be related to the increase in Pk values when the powders were dried. In this regard, PK has a physical implication for f values in the F-D data.
Lastly, in metronidazole preparations comprising drug/compacted-chitin matrix, the highly compactible tablets did not hinder the disintegration time and metronidazole immediate drug release. Such unaffected disintegration is more likely, as previously suggested, attributed to the presence of a larger mass of chitin material in compacted granules. The properties and dissolution of such preparation were even more favorable than tablets made of drug/MCC matrix.
It is evident that compaction results in a better flow of chitin powder and harder compacts. This may facilitate the utilization of such a powder without any further processing. Such processed powder is less liable to absorb water, as is the case for the ball milled chitin. Keeping the chitin excipient in a dry form allows it to be more appropriate for use in hot and humid climates where the drugs can be more stable when formulated with similar excipients. This behavior is unique when compared with other polymers, such as cellulose, where a balance between crystalline and amorphous states needs to be attained.