The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Liposomal Characteristics
2.2. Hydrogel Characteristics
2.3. Effect of Biological Fluids on The Texture Properties of Chitosan Hydrogels
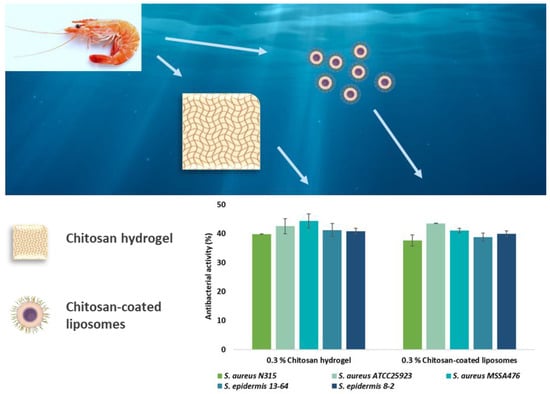
2.4. Antimicrobial Effects of Chitosan Formulations
3. Materials and Methods
3.1. Materials
3.2. Preparation of Liposomes
3.3. Vesicle Size Reduction
3.4. Chitosan Coating of Liposomes
3.5. Particle Size Analysis of Liposomes
3.6. Zeta Potential Measurements
3.7. Mucoadhesive Properties of Chitosan-Coated Liposomes
3.8. Hydrogel Preparation
3.9. Preparation of Biological Fluid Simulants
3.10. Determination of Texture Properties of Chitosan Hydrogels
3.11. Stability 0f Hydrogels in the Presence of Biological Fluid Simulants
3.12. Antibacterial Susceptibility Testing
3.13. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanić, Ž.; Škalko-Basnet, N. Hydrogels as intrinsic antimicrobials. In Hydrogels Based on Natural Polymers; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 309–328. [Google Scholar]
- Hurler, J.; Sørensen, K.K.; Fallarero, A.; Vuorela, P.; Skalko-Basnet, N. Liposomes-in-Hydrogel Delivery System with Mupirocin: In Vitro Antibiofilm Studies and In Vivo Evaluation in Mice Burn Model. BioMed. Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.; Bleher, S.; Flaten, G.E.; Tho, I.; Mattsson, S.; Skalko-Basnet, N. Chitosan in Mucoadhesive Drug Delivery: Focus on Local Vaginal Therapy. Mar. Drugs 2015, 13, 222–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanić, Ž.; Škalko-Basnet, N. Hydrogels for vaginal drug delivery. In Functional Hydrogels in Drug Delivery: Key Features and Future Perspectives, 1st ed.; Spizzirri, G., Cirillo, G., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 259–300. [Google Scholar]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta. Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-infective Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Kandimalla, K.K.; Borden, E.; Omtri, R.S.; Boyapati, S.P.; Smith, M.; Lebby, K.; Mulpuru, M.; Gadde, M. Ability of Chitosan Gels to Disrupt Bacterial Biofilms and Their Applications in the Treatment of Bacterial Vaginosis. J. Pharm. Sci. 2013, 102, 2096–2101. [Google Scholar] [CrossRef]
- Das Neves, J.; Amiji, M.; Sarmento, B. Mucoadhesive nanosystems for vaginal microbicide development: Friend or foe? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 389–399. [Google Scholar] [CrossRef]
- Pavelić, Ž.; Skalko-Basnet, N.; Schubert, R.; Jalšenjak, I. Liposomal Gels for Vaginal Drug Delivery. Methods Enzymol. 2004, 387, 287–299. [Google Scholar]
- Vanić, Ž.; Hurler, J.; Ferderber, K.; Golja Gasparovic, P.; Skalko-Basnet, N.; Filipovic-Grcic, J. Novel vaginal drug delivery system: Deformable propylene glycol liposomes-in-hydrogel. J. Liposome Res. 2014, 24, 27–36. [Google Scholar]
- Mesquita, L.; Galante, J.; Nunes, R.; Sarmento, B.; Das Neves, J. Pharmaceutical Vehicles for Vaginal and Rectal Administration of Anti-HIV Microbicide Nanosystems. Pharmaceutics 2019, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Liu, Q.; Wu, H.; Wang, K.; Li, L.; Zhou, C.; Ao, N. Preparation and characterization of in-situ formable liposome/chitosan composite hydrogels. Mater. Lett. 2018, 220, 289–292. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. [Google Scholar]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Şenyiğit, Z.A.; Karavana, S.Y.; Erac, B.; Gursel, O.; Limoncu, M.H.; Baloğlu, E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014, 64, 139–156. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Boil. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Yu, T.; Malcolm, K.; Woolfson, D.; Jones, D.S.; Andrews, G.P. Vaginal gel drug delivery systems: Understanding rheological characteristics and performance. Expert Opin. Drug Deliv. 2011, 8, 1309–1322. [Google Scholar] [CrossRef]
- Vanić, Ž; Skalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Jøraholmen, M.W.; Vanić, Ž.; Tho, I.; Skalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Karn, P.R.; Vanić, Z.; Pepić, I.; Škalko-Basnet, N. Mucoadhesive liposomal delivery systems: The choice of coating material. Drug. Dev. Ind. Pharm. 2011, 37, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Berginc, K.; Suljaković, S.; Skalko-Basnet, N.; Kristl, A. Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin. Eur. J. Pharm. Biopharm. 2014, 87, 40–46. [Google Scholar] [CrossRef]
- Takeuchi, H.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Effectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugs. Int. J. Pharm. 2005, 303, 160–170. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Bahia, M.F.; Amiji, M.M.; Sarmento, B. Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opin. Drug Deliv. 2011, 8, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Naderkhani, E.; Erber, A.; Škalko-Basnet, N.; Flaten, G.E. Improved Permeability of Acyclovir: Optimization of Mucoadhesive Liposomes Using the Phospholipid Vesicle-Based Permeation Assay. J. Pharm. Sci. 2014, 103, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Das Neves, J.; Bahia, M. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef]
- Boegh, M.; Foged, C.; Müllertz, A.; Nielsen, H.M. Mucosal drug delivery: Barriers, in vitro models and formulation strategies. J. Drug Deliv. Sci. Technol. 2013, 23, 383–391. [Google Scholar] [CrossRef]
- Millotti, G.; Hoyer, H.; Engbersen, J.; Bernkop-Schnürch, A. 6-mercaptonicotinamide-functionalized chitosan: A potential excipient for mucoadhesive drug delivery systems. J. Drug Deliv. Sci. Technol. 2010, 20, 181–186. [Google Scholar] [CrossRef]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Faccendini, A.; Puccio, A.; Caramella, C. Comparison of poloxamer-and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis. Drug. Dev. Ind. Pharm. 2014, 40, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, Ž.; Skalko-Basnet, N.; Jalšenjak, I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int. J. Pharm. 2005, 301, 140–148. [Google Scholar]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Cundell, A.M. Microbial ecology of the human skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. New Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Huebner, J.; Goldmann, D.A. Coagulase-negative staphylococci: Role as pathogens. Annu. Rev. Med. 1999, 50, 223–236. [Google Scholar] [CrossRef]
- Dumont, R.; Villet, M.; Guirand, A.; Montembault, A.; Delair, T.; Lack, S.; Barikosky, M.; Crepet, A.; Alcouffe, P.; Laurent, F.; et al. Processing and antimicrobial properties of chitosan-coated alginate fibers. Carbohydr. Polym. 2018, 190, 31–42. [Google Scholar] [CrossRef]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int. J. Boil. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Ng, I.S.; Lin, G.Y.; Chiu, C.Y.; Chang, Y.K. Antimicrobaila activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A Review of the Physical and Chemical Properties of Human Semen and the Formulation of a Semen Simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, J. Nephelometer:an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA 1907, 14, 1176–1178. [Google Scholar] [CrossRef] [Green Version]
- Heatley, N.G. A method for the assay of penicillin. Biochem. J. 1944, 38, 61–65. [Google Scholar] [CrossRef] [Green Version]







| Vesicle size | PI* | ||||
|---|---|---|---|---|---|
| Peak 1 (nm) | Weight Intensity (%) | Peak 2 (nm) | Weight Intensity (%) | ||
| Non-coated | 226 ± 10.2 | 89.2 | 55 ± 4.6 | 10.8 | 0.35 |
| 0.01 | 217 ± 0.7 | 90.2 | 49 ± 0.2 | 10.2 | 0.32 |
| 0.03 | 228 ± 2.4 | 90.5 | 54 ± 0.0 | 10.2 | 0.31 |
| 0.1 | 288 ± 71.1 | 74.9 | 75 ± 19.2 | 24.6 | 0.33 |
| 0.3 | 358 ± 90.8 | 63.5 | 106 ± 29.4 | 34.1 | 0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. https://0-doi-org.brum.beds.ac.uk/10.3390/md18020096
Jøraholmen MW, Bhargava A, Julin K, Johannessen M, Škalko-Basnet N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Marine Drugs. 2020; 18(2):96. https://0-doi-org.brum.beds.ac.uk/10.3390/md18020096
Chicago/Turabian StyleJøraholmen, May Wenche, Abhilasha Bhargava, Kjersti Julin, Mona Johannessen, and Nataša Škalko-Basnet. 2020. "The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation" Marine Drugs 18, no. 2: 96. https://0-doi-org.brum.beds.ac.uk/10.3390/md18020096