Childhood Health Status and Adulthood Cardiovascular Disease Morbidity in Rural China: Are They Related?
Abstract
:1. Introduction
2. Conceptual Framework
3. Materials and Methods
3.1. Data
3.2. Measures
3.2.1. Dependent Variables—Adult Cardiovascular Diseases
3.2.2. Independent Variables
3.3. Analytical Techniques
- Hadult = self-reported cardiovascular diseases;
- Hchild = childhood health status before 15 years old;
- X = a vector of other control variables;
- ε = a disturbance term; and
- β0β1β2 = coefficients to be estimated.
- IV = instrumental variables;
- u = residual;
- α0α1α2 = coefficients to be estimated.
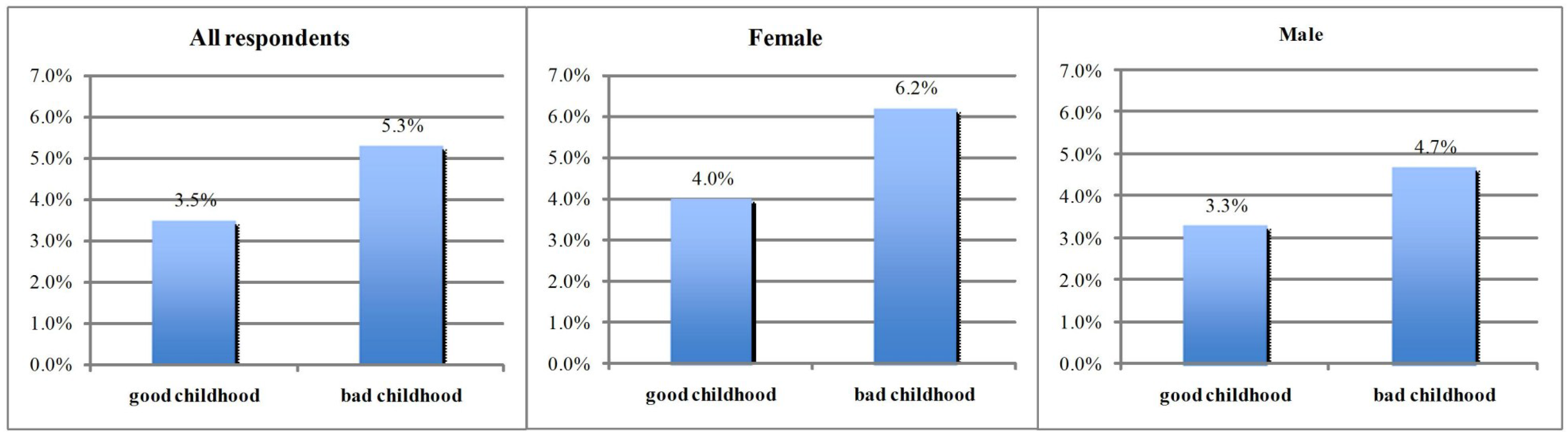
4. Results
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Center for Cardiovascular Diseases, China. Report on Cardiovascular Disease in China; Encyclopedia of China Publishing House: Beijing, China, 2014. [Google Scholar]
- Blackwell, D.L.; Hayward, M.D.; Crimmins, E.M. Does childhood health affect chronic morbidity in later life? Soc. Sci. Med. 2001, 52, 1269–1284. [Google Scholar] [CrossRef]
- Haas, S.A. The long-term effects of poor childhood health: An assessment and application of retrospective reports. Demography 2007, 44, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.L. Understanding the twentieth century decline in chronic conditions among older men. Demography 2000, 37, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Martyn, C.N.; Barker, D.J.P.; Osmond, C. Mothers’ pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet 1996, 348, 1264–1268. [Google Scholar] [CrossRef]
- Banks, J.; Oldfield, Z.; Smith, J.P. Childhood Health and Differences in Late-Life Health Outcomes between England and the United States. 2011. Available online: http://www.nber.org/papers/w17096 (accessed on 27 March 2016).
- Palloni, A.; Milesi, C.; White, R.G.; Turner, A. Early childhood health, reproduction of economic inequalities and the persistence of health and mortality differentials. Soc. Sci. Med. 2009, 68, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Portrait, F.; Teeuwiszen, E.; Deeg, D. Early life undernutrition and chronic diseases at older ages: The effects of the Dutch famine on cardiovascular diseases and diabetes. Soc. Sci. Med. 2011, 73, 711–718. [Google Scholar] [CrossRef] [PubMed]
- McEniry, M. Infant mortality, season of birth and the health of older Puerto Rican adults. Soc. Sci. Med. 2011, 72, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R. Childhood morbidity and health in early adulthood: Life course linkages in a high morbidity context. Adv. Life Course Res. 2010, 15, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Yao, S. A note on the causal factors of China’s famine in 1959–1961. J. Political Econ. 1999, 107, 1365–1369. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.A. The long-term health and economic consequences of the 1959–1961 famine in China. J. Health Econ. 2007, 26, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Song, S. Identifying the intergenerational effects of the 1959–1961 Chinese Great Leap Forward Famine on infant mortality. Econ. Hum. Biol. 2013, 11, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Ma, B. Socioeconomic status and obesity gradient over age: New evidence from China. Front. Econ. China 2007, 7, 70–93. [Google Scholar]
- Brandt, M.; Deindl, K.C.; Hank, K. Tracing the origins of successful aging: The role of childhood conditions and social inequality in explaining later life health. Soc. Sci. Med. 2012, 74, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, D.; Hayward, M.D. Early life influences on cognitive impairment among oldest old Chinese. J. Gerontol. Soc. Sci. 2008, 63B, S25–S33. [Google Scholar] [CrossRef]
- Case, A.; Fertig, A.; Paxson, C. The lasting impact of childhood health and circumstance. J. Health Econ. 2005, 24, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Soldo, B.; Elo, I.T. Do early-life conditions predict functional health status in adulthood? The case of Mexico. Soc. Sci. Med. 2011, 72, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Fetal nutrition and cardiovascular disease in later life. In Fetal and Early Childhood Environment: Long-Term Health Implications; Marmot, M.G., Wadsworth, M.E., Eds.; The Royal Society of Medicine Press: London, UK, 1997; pp. 96–108. [Google Scholar]
- Lithell, H.O.; McKeigue, P.M.; Berglund, L.; Mohsen, R.; Lithell, U.B.; Leon, D.A. Relation of size at birth to non-insulin dependent diabetes insulin concentrations in men aged 50–60 years. Br. Med. J. 1996, 312, 406–410. [Google Scholar] [CrossRef]
- Newsome, C.A.; Shiell, A.W.; Fall, C.H.; Phillips, D.I.; Shier, R.; Law, C.M. Is birth weight related to later glucose insulin metabolism? A systematic review. Diabet. Med. 2003, 20, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J. Development of the endocrine pancreas. Rev. Endocr. Metab. Disord. 2005, 6, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The Fetal and infant origins of adult disease. Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef]
- Nastis, S.A.; Crocker, T.D. Valuing mother and child health: The intrauterine environment. Econ. Hum. Biol. 2012, 10, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Kuh, D.; Shlomo, Y.B. A Life Course Approach to Chronic Disease Epidemiology; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Li, Y.; Schouten, E.; Hu, X.; Cui, Z.; Luan, D.; Ma, G. Obesity prevalence and time trend among youngsters in China, 1982–2002. Asia Pac. J. Clin. Nutr. 2008, 17, 131–137. [Google Scholar] [PubMed]
- Ozanne, S.E.; Hales, C.N. Lifespan: Catch-up growth and obesity in male mice. Nature 2004, 427, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.E.; Crimmins, E.M. Inflammatory exposure and historical changes in human life-spans. Science 2004, 305, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Finch, C.E. Infection, inflammation, height, and longevity. Proc. Natl. Acad. Sci. USA 2006, 103, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Grantham-McGregor, S.; Ani, C. A review of studies on the effect of iron deficiency on cognitive development in children. J. Nutr. 2001, 131, 649S–668S. [Google Scholar] [PubMed]
- Black, D.; Morris, J.N.; Smith, C.; Townsend, P.; Whitehead, M. Inequalities in Health: The Black Report. The Health Divide; Penguin: London, UK, 1998. [Google Scholar]
- Heck, K.E.; Parker, J.D. Family structure, socioeconomic status, and access to health care for children. Health Serv. Res. 2002, 37, 173–186. [Google Scholar] [PubMed]
- Contoyannis, P.; Jones, A.M. Socio-economic status, health and life style. J. Health Econ. 2004, 23, 965–995. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.W.; Kaplan, G.A.; Salonen, J.T. Why do poor people behave poorly? Variation in adult health behaviors and psychosocial characteristics by stages of the socioeconomic life course. Soc. Sci. Med. 1997, 44, 809–819. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Borooah, V.K. Logit and Logit: Ordered and Multinomial Models; Sage: New York, NY, USA, 2002. [Google Scholar]
- Wooldridge, J.M. Econometric Analysis of Cross Section and Panel Data; The MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Lin, J.Y. Collectivization and China’s agricultural crisis in 1959–1961. J. Political Econ. 1990, 98, 1228–1252. [Google Scholar] [CrossRef]
- Terzaa, J.; Anirban, B.; Paul, J.R. Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J. Health Econ. 2008, 27, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Gourieroux, C. Generalized residuals. J. Econom. 1987, 34, 5–32. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap methods: Another look at the jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Smith, J.P. Reconstructing Childhood Health Histories. Demography 2009, 46, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Shen, Y.; Strauss, J.; Zhe, Y.; Zhao, Y. The effects of childhood health on adult health and SES in China. Econ. Dev. Cult. Chang. 2012, 61, 127–155. [Google Scholar] [CrossRef] [PubMed]
| Factor | All Respondents (n = 5735) | Male (n = 2764) | Female (n = 2971) |
|---|---|---|---|
| Morbidity of cardiovascular diseases | 4.17% | 4.5% | 3.8% |
| Good childhood health | 72.5% | 72.9% | 72.02% |
| Education level | |||
| Primary school and below | 69.1% | 67.6% | 70.7% |
| Junior high school | 21.6% | 27.5% | 15.3% |
| High school and above | 9.3% | 12.5% | 5.9% |
| Household income per capita (1000 Yuan) # | 8.49 (18.86) | 8.91 (18.92) | 8.16 (18.45) |
| Hypertension | 19.9% | 18.5% | 21.4% |
| Dyslipidemia | 7.29% | 6.8% | 7.84% |
| Age | 54.91 | 55.03 | 54.66 |
| Gender | 48.2% | ||
| Marital status | 92.7% | 93.6% | 91.7% |
| Smoker | 30.06% | 53.3% | 5.1% |
| Frequent drinker | 26.99% | 45.9% | 6.7% |
| Factor | All Respondents | Male | Female | |||
|---|---|---|---|---|---|---|
| 2SRI Coef. (Std.) | Probit Coef. (Std.) | 2SRI Coef. (Std.) | Probit Coef. (Std.) | 2SRI Coef. (Std.) | Probit Coef. (Std.) | |
| Good childhood health | −2.10 *** | −0.24 *** | −2.59 * | −0.27 *** | −1.73 ** | −0.20 ** |
| (0.74) | (0.06) | (1.38) | (0.09) | (0.06) | (0.09) | |
| Residual | 1.13 ** | - | 1.38 * | - | 0.99 * | - |
| (0.44) | - | (0.82) | - | (0.51) | - | |
| Household income | −0.01 | −0.01 | −0.01 | −0.01 | −0.01 | −0.01 |
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |
| Dyslipidemia | 0.21 * | 0.31 *** | 0.22 | 0.42 *** | 0.09 | 0.17 |
| (0.11) | (0.11) | (0.16) | (0.15) | (0.24) | (0.18) | |
| Hypertension | 0.28 | 0.1 * | 0.27 | 0.01 | 0.28 * | 0.22 * |
| (0.22) | (0.08) | (0.29) | (0.26) | (0.15) | (0.11) | |
| Age | −0.001 | 0.20 | −0.002 | −9.67 × 10−5 | 0.15 | 0.46 * |
| (0.002) | (0.19) | (0.002) | (0.002) | (0.37) | (0.28) | |
| Age quadric | −0.001 | −0.002 | −0.001 | −0.001 | −0.003 | −0.004 |
| (0.001) | (0.001) | (0.002) | (0.002) | (0.003) | (0.003) | |
| Gender | −0.0297 | 0.150 * | - | - | - | - |
| (0.0945) | (0.0906) | - | - | - | - | |
| Junior high school | 0.01 | −0.03 | 0.02 | 0.29 | −0.03 | −0.05 |
| (0.09) | (0.08) | (0.18) | (0.18) | (0.13) | (0.10) | |
| High school | 0.12 | 0.03 | 0.2 | 0.50 *** | 0.09 | −0.10 |
| (0.14) | (0.11) | (0.21) | (0.16) | (0.15) | (0.13) | |
| Marital status | 0.08 | 0.02 | 0.08 | 0.08 | 0.09 | 0.01 |
| (0.10) | (0.13) | (0.17) | (0.16) | (0.23) | (0.19) | |
| Smoker | −0.17 * | −0.002 | 0.25 | 0.29 | −0.18 | −0.12 |
| (0.09) | (0.08) | (0.19) | (0.18) | (0.11) | (0.08) | |
| Frequent drinker | 0.14 | 0.45 | −0.01 | −0.07 | 0.85 ** | 0.46 |
| (0.11) | (0.34) | (0.23) | (0.18) | (0.42) | (0.36) | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Shen, J.J. Childhood Health Status and Adulthood Cardiovascular Disease Morbidity in Rural China: Are They Related? Int. J. Environ. Res. Public Health 2016, 13, 565. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060565
Wang Q, Shen JJ. Childhood Health Status and Adulthood Cardiovascular Disease Morbidity in Rural China: Are They Related? International Journal of Environmental Research and Public Health. 2016; 13(6):565. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060565
Chicago/Turabian StyleWang, Qing, and Jay J. Shen. 2016. "Childhood Health Status and Adulthood Cardiovascular Disease Morbidity in Rural China: Are They Related?" International Journal of Environmental Research and Public Health 13, no. 6: 565. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060565





