Fabrication of Stabilized Fe–Mn Binary Oxide Nanoparticles: Effective Adsorption of 17β-Estradiol and Influencing Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CMC-FMBON
2.3. Characterization of CMC-FMBON
2.4. E2 Removal from Aqueous Solution: Batch Adsorption Tests
3. Results and Discussion
3.1. Characterization of FMBON and CMC-FMBON
3.2. The Removal Capacity of CMC-FMBON for E2
3.2.1. Stabilizers Content
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherm Experiment and Thermodynamic
3.3. The External Environmental Factors on Removal Process of E2
3.3.1. Effect of Initial Solution pH on E2 Removal Process
3.3.2. Effect of Ionic Strength and Coexisting Ions on E2 Removal
3.3.3. Effect of Humic Acid on E2 Removal
3.3.4. Comparisons with the Other Materials
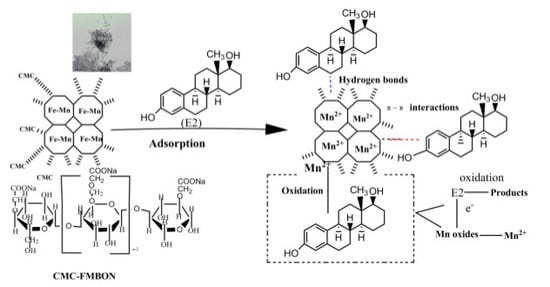
3.3.5. Adsorption Mechanism of CMC-FMBON for E2
3.3.6. Desorption and Regeneration Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A.; Antonopoulou, M.; Konstantinou, I.K. Removal of Endocrine Disrupting Chemicals in HSF and VF pilot-scale constructed wetlands. Chem. Eng. J. 2016, 294, 146–156. [Google Scholar] [CrossRef]
- Fukuhara, T.; Iwasaki, S.; Kawashima, M.; Shinohara, O.; Abe, I. Absorbability of estrone and 17beta-estradiol in water onto activated carbon. Water Res. 2006, 40, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.M.; Liu, Y.G.; Liu, S.B.; Jiang, L.H.; Zeng, G.M.; Zeng, Z.W.; Wang, X.H.; Li, J.; Kare, Z. Fabrication of hydrochar functionalized Fe–Mn binary oxide nanocomposites: Characterization and 17β-estradiol removal. RSC. Adv. 2017, 7, 37122–37129. [Google Scholar] [CrossRef]
- Scsukova, S.; Rollerova, E.; Bujnakova Mlynarcikova, A. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer. Reprod. Boil. 2016, 16, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, K.L.; Maack, G.; Benstead, R.; Tyler, C.R. Estrogenic Wastewater Treatment Works Effluents Reduce Egg Production in Fish. Environ. Sci. Technol. 2009, 43, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Wang, Q.; Wang, C.; Wang, P.; Mao, K. Performance evaluation and application of surface-molecular-imprinted polymer-modified TiO2 nanotubes for the removal of estrogenic chemicals from secondary effluents. Environ. Sci. Pollut. Res. Int. 2013, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, J. Photo-Fenton degradation of 17β-estradiol in presence of α-FeOOHR and H2O2. Appl. Catal. B Environ. 2008, 78, 250–258. [Google Scholar]
- Xie, W.; Zhao, D. Controlling phosphate releasing from poultry litter using stabilized Fe–Mn binary oxide nanoparticles. Sci. Total Environ. 2016, 542, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Liu, Y.G.; Zeng, G.M.; Liu, S.B.; Hu, X.J.; Shu, D.; Jiang, L.H.; Tan, X.F.; Cai, X.X.; Yan, Z.L. Tetracycline absorbed onto nitrilotriacetic acid-functionalized magnetic graphene oxide: Influencing factors and uptake mechanism. J. Colloid Interface Sci. 2017, 485, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-F.; Liu, Y.-G.; Liu, S.-B.; Shu, D.; Zeng, G.-M.; Hu, X.-J.; Tan, X.-F.; Jiang, L.-H.; Yan, Z.-L.; Cai, X.-X. Cu(II)-influenced adsorption of ciprofloxacin from aqueous solutions by magnetic graphene oxide/nitrilotriacetic acid nanocomposite: Competition and enhancement mechanisms. Chem. Eng. J. 2017, 319, 219–228. [Google Scholar] [CrossRef]
- Bradley, P.M.; Writer, J.H. Effect of Light on Biodegradation of Estrone, 17β-Estradiol, and 17α-Ethinylestradiol in Stream Sediment. J. Am. Water. Resour. A 2014, 50, 334–342. [Google Scholar] [CrossRef]
- Jiang, L.H.; Liu, Y.G.; Zeng, G.M.; Xiao, F.Y.; Hu, X.J.; Hu, X.; Wang, H.; Li, T.T.; Zhou, L.; Tan, X.F. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
- Jiang, L.H.; Liu, Y.G.; Liu, S.B.; Hu, X.J.; Zeng, G.M.; Hu, X.; Liu, S.M.; Liu, S.H.; Huang, B.Y.; Li, M.F. Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem. Eng. J. 2017, 308, 597–605. [Google Scholar] [CrossRef]
- Yang, Z.H.; Xiong, S.; Wang, B.; Li, Q.; Yang, W.C. Cr(III) adsorption by sugarcane pulp residue and biochar. J. Cent. South. Univ. 2013, 20, 1319–1325. [Google Scholar] [CrossRef]
- Jia, C.-G.; Zhang, Y.-P.; Wang, H.; Ou, G.-N.; Liu, Q.-M.; Lin, J.-M. Rapid biosorption and reduction removal of Cr(VI) from aqueous solution by dried seaweeds. J. Cent. South Univ. 2014, 21, 2801–2809. [Google Scholar] [CrossRef]
- Cai, H.-M.; Chen, G.-J.; Peng, C.-Y.; Zhang, Z.-Z.; Dong, Y.-Y.; Shang, G.-Z.; Zhu, X.-H.; Gao, H.-J.; Wan, X.-C. Removal of fluoride from drinking water using tea waste loaded with Al/Fe oxides: A novel, safe and efficient biosorbent. Appl. Surf. Sci. 2015, 328, 34–44. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Lin, X.; Su, X.; Zhang, Y. Removal of fluoride from groundwater by adsorption onto La(III)-Al(III) loaded scoria adsorbent. Appl. Surf. Sci. 2014, 303, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J. Removal of estrone and 17β-estradiol from water by adsorption. Water Res. 2005, 39, 3991–4003. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Esparza, M. HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003, 37, 3530–3537. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, C.; Xu, N.; Ni, J. Effect of inorganic nanoparticles on 17β-estradiol and 17α-ethynylestradiol adsorption by multi-walled carbon nanotubes. Environ. Pollut. 2015, 205, 111–120. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Liang, Q.; Zhao, D. Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Res. 2011, 45, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.T.; Kuan, W.H.; Chen, T.Y.; Wang, M.K. Adsorption mechanism of selenate and selenite on the binary oxide systems. Water Res. 2009, 43, 4412–4420. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Kang, J.; Fan, M.; Qu, J. Removal of tetracycline from water by Fe-Mn binary oxide. J. Environ. Sci. 2012, 24, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe–Mn binary oxide adsorben. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, D.; Liu, J.; Roberts, C.B. Stabilization of Fe-Pd nanoparticles with Sodium Carboxymethyl Cellulose for Enhanced Transport and Dechlorination of Trichlororthylene in Soil and Groundwater. Ind. Eng. Chem. Res. 2007, 46, 29–34. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Khan, M.R.; Alothman, Z.A. Adsorptive Removal of Toxic Dye Using Fe3O4–TSC Nanocomposite: Equilibrium, Kinetic, and Thermodynamic Studies. J. Chem. Eng. Data 2016, 61, 3806–3813. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Abdullah Alothman, Z.; Alshehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel Metal-Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th(IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhao, D. Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles. J. Hazard Mater. 2012, 211–212, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, K. Adsorption of 17β-estradiol by multi-walled carbon nanotubes in natural waters with or without aquatic colloids. Chem. Eng. J. 2014, 258, 185–193. [Google Scholar] [CrossRef]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Li, G.T. Removal Mechanism of As(III) by a Novel Fe-Mn Binary Oxide Adsorbent: Oxidation and Sorption. Environ. Sci.Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, X.; Zhang, H.; Chen, C.; Wang, X. Effect of environmental conditions on Pb(II) adsorption on β-MnO2. Chem. Eng. J. 2010, 164, 49–55. [Google Scholar] [CrossRef]
- Allard, S.; von Gunten, U.; Sahli, E.; Nicolau, R.; Gallard, H. Oxidation of iodide and iodine on birnessite (δ-MnO2) in the pH range 4–8. Water Res. 2009, 43, 3417–3426. [Google Scholar] [CrossRef] [PubMed]
- Eusterhues, K.; Wagner, F.E.; Häusler, W.; Hanzlik, M.; Knicker, H.; Totsche, K.U.; Kögel-Knabner, I.; Schwertmann, U. Characterization of Ferrihydrite-Soil Organic Matter Coprecipitates by X-ray Diffraction and Mossbauer Spectroscopy. Environ. Sci. Technol. 2008, 42, 7891–7897. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-L.; Liu, Y.-G.; Tan, X.-F.; Liu, S.-B. Immobilization of aqueous and sediment-sorbed ciprofloxacin by stabilized Fe-Mn binary oxide nanoparticles: Influencing factors and reaction mechanisms. Chem. Eng. J. 2017, 314, 612–621. [Google Scholar] [CrossRef]
- Unur, E. Functional nanoporous carbons from hydrothermally treated biomass for environmental purification. Microporous Mesoporous Mater. 2013, 168, 92–101. [Google Scholar] [CrossRef]
- Zhou, J.; Song, H.; Ma, L.; Chen, X. Magnetite/graphene nanosheet composites: Interfacial interaction and its impact on the durable high-rate performance in lithium-ion batteries. RSC Adv. 2011, 1, 782. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem.Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liang, Q.; Qian, T.; Zhao, D. Immobilization of selenite in soil and groundwater using stabilized Fe-Mn binary oxide nanoparticles. Water Res. 2015, 70, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, R.; Arul Jothi, N.; Jayavignesh, V.; Karthikaiselvi, K.; Antony, G.I.; Sharmila, P.R.; Kavitha, S.; Swaminathan, K. As(V) removal using carbonized yeast cells containing silver nanoparticles. Water Res. 2011, 45, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.X.; Chen, A.W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoon, J.; von Gunten, U. Kinetics of the Oxidation of Phenols and Phenolic Endocrine Disruptors during Water Treatment with Ferrate (Fe(VI)). Environ. Sci. Technol. 2005, 39, 8978–8984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.; Karanfil, T. Adsorption of synthetic organic chemicals by carbon nanotubes: Effects of background solution chemistry. Water Res. 2010, 44, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic propeties of aquatic humic substances. Anal. Chim. Acta 1966, 337, 133–149. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal ion Pollution Management. Environ. Sci. Technol 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Zaib, Q.; Khan, I.A.; Saleh, N.B.; Flora, J.R.V.; Park, Y.-G.; Yoon, Y. Removal of Bisphenol A and 17β-Estradiol by Single-Walled Carbon Nanotubes in Aqueous Solution: Adsorption and Molecular Modeling. Water Air Soil Pollut. 2012, 223, 3281–3293. [Google Scholar] [CrossRef]
- Rovani, S.; Rodrigues, A.G.; Medeiros, L.F.; Cataluña, R.; Lima, É.C.; Fernandes, A.N. Synthesis and characterisation of activated carbon from agroindustrial waste—Preliminary study of 17β-estradiol removal from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2128–2137. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Zhao, M.; Qiu, Y.; Sheng, G.D. Oxidative removal of aqueous steroid estrogens by manganese oxides. Water Res. 2008, 42, 5038–5044. [Google Scholar] [CrossRef] [PubMed]








| Adsorbent | qe. exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| R2 | qe (mg/g) | K1 (g/mg·min) | R2 | qe (mg/g) | K2 (g/mg·min) | ||
| CMC-FMBON | 84.20 | 0.96 | 80.47 | 0.03 | 0.98 | 82.23 | 0.04 |
| FMBON | 60.32 | 0.96 | 63.28 | 0.04 | 0.98 | 55.21 | 0.41 |
| Adsorbent | T | Langmuir Isotherm | Freundich Isotherm | |||||
|---|---|---|---|---|---|---|---|---|
| KL | qe | R2 | KF | qe | n | R2 | ||
| CMC-FMBON | 298 K | 0.20 | 124.10 | 0.98 | 55.58 | 142.30 | 1.11 | 0.93 |
| 318 K | 0.14 | 112.22 | 0.98 | 29.32 | 125.02 | 1.23 | 0.93 | |
| 338 K | 0.10 | 100.52 | 0.98 | 20.60 | 98.01 | 1.10 | 0.95 | |
| FMBON | 298 K | 0.39 | 98.14 | 0.97 | 50.12 | 95.21 | 0.96 | 0.91 |
| 318 K | 0.12 | 84.41 | 0.99 | 25.33 | 81.24 | 0.95 | 0.96 | |
| 338 K | 0.08 | 75.21 | 0.98 | 15.48 | 78.32 | 1.02 | 0.94 | |
| Adsorbents | Temperature (T) | ln k0 | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/K·mol) | R2 |
|---|---|---|---|---|---|---|
| CMC-FMBON | 298 K | 1.21 | −2.24 | −8.846 | −12.27 | 0.96 |
| 318 K | 1.04 | −1.91 | ||||
| 338 K | 1.12 | −1.82 | ||||
| FMBON | 298 K | 0.811 | −2.15 | −7.65 | −20.12 | 0.97 |
| 318 K | 0.696 | −1.74 | ||||
| 338 K | 0.511 | −1.12 |
| Adsorbent | Adsorption Capacity (qe (mg/g)) | References |
|---|---|---|
| Few-layered graphene oxide nanosheets | 149.9 | [12] |
| Sing-walled carbon nanotubes | 27.2 | [50] |
| Activated carbons | 21.3–67.6 | [2] |
| Magnetic graphene oxide | 85.80 | [13] |
| hydrochar-FMBO | 49.77 | [3] |
| ACM (activated carbon purchased from Merck) | 5.07 | [51] |
| CS (coffee waste and sawdust) | 4.95 | [51] |
| CMC-FMBON | 124.10 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Q.; Yin, Z.; Liu, Y.; Tan, X.; Zeng, G.; Jiang, L.; Liu, S.; Tian, S.; Liu, N.; Wang, X. Fabrication of Stabilized Fe–Mn Binary Oxide Nanoparticles: Effective Adsorption of 17β-Estradiol and Influencing Factors. Int. J. Environ. Res. Public Health 2018, 15, 2218. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15102218
Ning Q, Yin Z, Liu Y, Tan X, Zeng G, Jiang L, Liu S, Tian S, Liu N, Wang X. Fabrication of Stabilized Fe–Mn Binary Oxide Nanoparticles: Effective Adsorption of 17β-Estradiol and Influencing Factors. International Journal of Environmental Research and Public Health. 2018; 15(10):2218. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15102218
Chicago/Turabian StyleNing, Qimeng, Zhihong Yin, Yunguo Liu, Xiaofei Tan, Guangming Zeng, Luhua Jiang, Shaobo Liu, Sirong Tian, Ni Liu, and Xiaohua Wang. 2018. "Fabrication of Stabilized Fe–Mn Binary Oxide Nanoparticles: Effective Adsorption of 17β-Estradiol and Influencing Factors" International Journal of Environmental Research and Public Health 15, no. 10: 2218. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15102218