3.1. Characterization of the Raw ASR
The characterization of the raw HB-ASR was selected as a representative to illustrate. The characterization of the raw HB-ASR is presented in
Figure 1. As shown in
Figure 1a,b,d, the general particles were irregular in shape, and sulfur (S
8) crystal structures were found. The amorphous particles agglomerate into large particles. The EDS results showed that the residue was mainly composed of S and As, indicating that the amorphous particles are As-S compounds.
Figure 1d shows that the three broad peaks occur at 2θ values near 18°, 31° and 57°, similar to that of amorphous As
2S
3, as reported [
35].
Figure 1c shows a wide range of particle size distribution from 0.5 μm to 60 μm. The size of the particles was distributed in four concentrated areas of approximately 0.52 μm, 4.24 μm, 16.11 μm and 34.57 μm. The median particle size (D
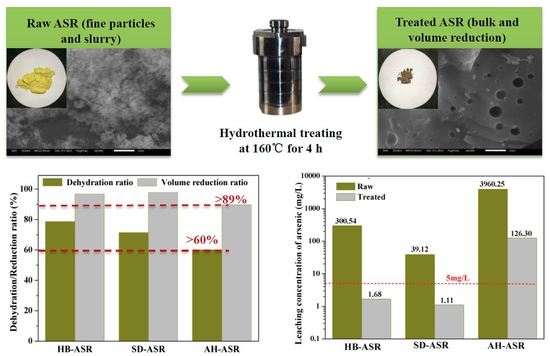
50) of 6.23 μm indirectly reflected that most small particles were agglomerated into large particles. A leaching test was performed using the TCLP method, and the results indicated that the arsenic-leachate concentration was 300.54 mg/L, which is far greater than the regulation limit of 5 mg/L for As.
3.3. Variations in Heavy Metal Contents
Table 4 shows the heavy metal contents in each ASR before and after treatment. After hydrothermal treatment, the contents of As and Cu increased somewhat, whereas the contents of Cd, Cr, Pb and Zn were slightly reduced. This might be attributed to the different behaviors of these heavy metals during the treatment. For example, some adsorbed ions could be washed off, while some sulfides could decompose into soluble salts and volatile H
2S [
37].
Table 5 shows the heavy metals concentration of the hydrothermal solution during the treatment process. Clearly, it can be seen that a large amount of heavy metals were dissolved in the hydrothermal process under high temperature and high pressure, thereby changing the content of heavy metals in the solidified body.
Since the hydrothermal fluid contained high concentrations of heavy metals, it was necessary to assess the stability of heavy metals in the hydrothermal process and avoid the transfer of contaminants into the wastewater.
Figure 2 shows the stabilization ratio for ASRs over the hydrothermal temperature at 160 °C for 4 h. As can be seen from
Figure 2, the stabilization ratios of heavy metals from high to low were copper, lead, arsenic, cadmium, chromium and zinc, respectively. The stabilization ratios of arsenic, copper and lead were close to 100%, while most of the chromium and zinc were dissolved in the hydrothermal fluid during the dehydration process. It can be seen that the amount of secondary pollutants produced by the hydrothermal fluid was relatively small, and the impact on the quality of heavy metals in the solidified ASR block is not significant.
3.5. Chemical Speciation of Heavy Metals
Three-staged BCR sequential extraction is conducted to assess environmental activity and potential ecological risks. Generally, the arsenic and other heavy metals in acid soluble and reducible fraction is classified as direct effect phases for environmental availability and ecological risk because they are presented as a loosely bound phase or thermodynamically unstable phase, respectively, which are likely to release into the environment. Meanwhile, arsenic associated with the oxidizable fraction is identified as a potential effect fraction because it can be liberated or transformed into an acid soluble and reducible fraction under oxidizing conditions. Only the residual fraction is believed to be a stable fraction because it contains mainly primary and secondary minerals, which may retain metal elements within their crystal or glass structure [
29].
The relative percentage of As and other heavy metals extracted in the different steps of the BCR test is presented in
Table 7. After the hydrothermal treatment, the acid soluble, reducible and oxidizable soluble fractions of arsenic dramatically decrease from 1.16%, 0.08% and 90.00% to 0.01%, 0.01% and 36.23%, respectively, indicating that direct toxicity effect fractions are reduced [
30]. The acid soluble, reducible and oxidizable soluble states of other heavy metals (Cd, Cr, Cu and Zn) also significantly decreased. In terms of residual fractions, the arsenic and other heavy metals (Cd, Cr, Cu, Pb and Zn) of them increased 54.99%, 36.82%, 15.48%, 25.65%, 10.37%, and 43.63%, respectively. Thus, an important conclusion is that the chemical species of arsenic and other heavy metals in sludge are significantly transformed to residual fractions by the hydrothermal treatment, resulting in a restrained environmental availability.
3.6. Phase Transformation
Figure 3 shows the XRD patterns of ASRs before and after treatment. As it can be seen from
Figure 3, the ASRs before and after treatment were mainly amorphous with only a few diffraction peaks. Only the peaks of arsenic trioxide were found in the raw AH-ASR, probably due to the oxidation of the waste residue during the long-term storage. The crystalline form of sulfur (S
8) was observed in the raw HB-ASR, which might be attributed to the reaction of S
2− and SO
32− in the previous waste acid treatment process. No diffraction peaks were found in SD-ASR.
Moreover, for AH-ASR, As
2O
3 species were apparently detected in the raw material and the intensity of the As
2O
3 peaks increased somewhat after treatment. This could explain the decline in the As leaching concentration as the better crystalline structure meant a lower contact area with the leaching agent. On the other hand, it could be found that the crystalline sulfur (S
8) in HB-ASR completely disappeared after the treatment. During the hydrothermal process, the sulfur (S
8) could decompose into H
2S by disproportionation reaction [
38]. The H
2S gas could precipitate heavy metals and thus reduced their leaching concentrations. For SD-ASR, it was amorphous and no changes on its XRD pattern could be observed after the treatment. Surprisingly, no new crystalline phase of arsenic sulfide compounds, such as realgar and orpiment, were found after hydrothermal treatment. This is probably because under these conditions it was difficult to generate crystalline As
2S
3 (c-As
2S
3) [
39,
40]. Although the XRD analysis results can explain the decrease in the leaching concentrations of some metals, it is insufficient and the reason for dehydration and volume reduction may be related to the morphology of the ASRs, which will be discussed in the following section.
3.7. Morphology Change
The SEM images of treated HB-ASR were selected as a representative to illustrate the morphology changes during hydrothermal treatment.
Figure 4 shows that there are obvious differences in the morphology of the HB-ASR with different treatment times. Before treatment, the ASR was flocculent particles that were composed of extremely fines of arsenic sulfide (
Figure 4a,b). After being treated for 120 min, the flocculent particles bonded together and formed a network of a porous body (
Figure 4c,d). In our experiments, it was found that the product generated under this condition was very easily crushed. Finally, a large bulk with a smooth surface was obtained after 240 min (
Figure 4e,f). There were some spherular pits on the fracture surface of the bulk, which might be caused byH
2S gas generated by sulfur decomposition. Because the amorphous and flocculent ASR was converted into a large bulk, the water content and volume of ASR reduced dramatically. This phenomenon was also found in the process of coal treatment by a hydrothermal method [
35]. In summary, the densification of ASRs in the hydrothermal process is the main reason for volume reduction, dehydration and stabilization/solidification.
3.8. The Analysis of Raman and XPS
The Raman technique was further applied to investigate the structural variation in the treated HB-ASR (
Figure 5). In the raw ASR, four peaks located at approximately ~153, ~219, ~340, and ~474 cm
−1 were found. The peak at ~340 cm
−1 indicated the existence of arsenic(III) sulfide [
41,
42]. The others (~153, ~219, and ~474 cm
−1) suggested the presence of sulfur [
43]. After the sample was treated for 120 min, the peak at 362 cm
−1 was found and the intensity became stronger after 240 min, indicating that arsenic(II) sulfide became clearly observable [
44,
45,
46]. The trend of the peak (~340 cm
−1) shifting to peak (~362 cm
−1) was probably a reflection of the decomposition of arsenic(III) sulfide into arsenic(II) sulfide.
XPS survey-spectra of the raw HB-ASR and sample treated for 240 min are shown in
Figure 6. It indicates the presence of S, As and O. It was obvious that the intensity of peak O 1s was lower after the treatment. The As 3d and S 2p spectra for the raw and treated sample are presented in
Figure 7. The Gaussian-Lorentzian resolving was performed to analyse the components of the sample [
47]. Raw spectra were fitted using a least-squares procedure with peaks of convoluted Gaussian (80%) and Lorentzian (20%) peak shape after subtraction of a Shirley baseline. The S 2p spectra were modeled as doublets of 2p
1/2 and 2p
3/2, separated by 1.2 eV and the area of the S 2p
1/2 peak was half the area of S 2p
3/2 peak. The As 3d spectra were modeled as doublets of 3d
3/2 and 3d
5/2, separated by 0.7 eV. The area of the As 3d
3/2 peak was two-thirds the area of the As 3d
5/2 peak [
48]. A higher binding energy is indicative of a higher oxidation state of arsenic and a lower binding energy corresponds to a lower oxidation state.
The surface compositions of sulfur are shown in
Figure 7 and
Table 8. The major peaks of the S 2p
3/2 spectrum of raw ASR were located at 162.80 and 164.02 eV, which were assigned to orpiment-like S
2− and S(0), respectively. The reported precipitates of arsenic sulfide formed in the As(III) removing process showed the S 2p
3/2 binding energies of 162.6 eV and 163.1 eV, which were assigned to the orpiment-like sulfide ion of S-As(III) and realgar-like sulfide ion of S-As(II), respectively [
49,
50]. As shown in
Table 6, after the treatment, the atom ratio of S species increased from 67% to 71%. In addition, the S(0) species accounted for 49% of the total sulfur content in the raw ASR and it increased to 54% after treatment. Finally, it is noted that the realgar-like sulfide ion of S-As(II) also increased to 11%.
The binding energy of the As 3d
5/2 peak is fitted with three As components consisting of As(III) and As(II). The reported peaks of As 3d
5/2 at 43.1, 43.4 and 44.8 eV were attributed to As(II)-S, As(III)-S and As(III)-O, respectively [
4,
27,
48,
51]. As shown in
Table 6, before the treatment, the content of As(III)-S (72%) predominated the As speciation, followed by As(III)-O (28%). However, the content of As(III)-S decreased to 70% and new As(II)-S reached to 17% after the hydrothermal treatment. This change was consistent with results of Raman analysis. On the other hand, Gallegos et al. [
52] reported it is thermodynamically favorable for As(III) sulfide to decompose into As
4S
4-like phase and S under reducing conditions. Hence, it can be reasoned that the hydrophobic sulfur melts to a liquid to encapsulate the arsenic sulfide compounds, making the particles bond together during the hydrothermal process and thus resulting in the dehydration, volume reduction and S/S of heavy metals.
Based on the SEM, Raman and XPS results, it is believed that the hydrophobic sulfur (S
0) and reaction of As(III)-S had a significant influence on the densification of ASR. The schematic diagram for this mechanism is illustrated in
Figure 8. First, As(III) sulfide generates hydrophobic sulfur and As(II) sulfide under reductive conditions. Second, the fine particles of As(III) sulfide or As(II) sulfide were bound together by the melted sulfur. Finally, the small formed pieces grew into a larger ASR bulk under high pressure due to adhesion by sulfur. The hydrophobicity of sulfur might be the reason for the satisfactory results of dehydration and volume reduction.