On the Kinetics of Degradation Reaction Determined Post Accelerated Weathering of Polyolefin Plastic Waste Blends
Abstract
:1. Introduction
1.1. Dependency on Plastics and Environmental Pollution
1.2. Recovery and Valorization of Plastics
2. Materials and Methods
2.1. Materials Acquirement and Samples Compounding/Recycling
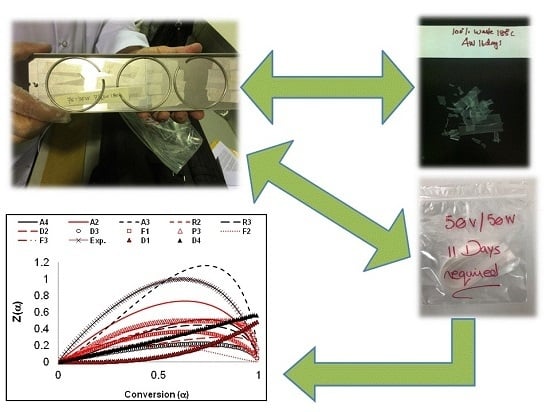
2.2. Accelerated Weathering
2.3. Differential Scanning Calorimetry (DSC)
2.4. Thermogravimetric Analysis (TGA)
2.5. Kinetics of Degradation Analysis and Mathematical Modeling Framework
3. Results and Discussion
3.1. Crystallinity and Thermal Stability
3.2. Model Free Kinetics Methods and Master Curve Study
3.3. Analytical Solution Model
3.4. Implications in the Context of Plastic Pollution
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| Ao | Pre-Exponential Factor (min−1) |
| BPA | Bisphenol-A |
| DEHP | Di-(2-ethylhexyl)phthalate |
| DSC | Differential Scanning Calorimeter |
| Ea | Apparent Activation Energy (kJ·mol−1) |
| EU | European Union |
| f(α) | Reaction Model |
| FWO | Flynn-Wall-Ozawa Isoconversion Method |
| GDP | Gross Domestic Product |
| GHGs | Greenhouse Gases |
| HDPE | High Density Polyethylene |
| LDPE | Low Density Polyethylene |
| LLDPE | Linear Low Density Polyethylene |
| m | Mass of Polymeric Material at a Specific Time of Reaction |
| ME | Middle East |
| mf | Final Mass of Polymer |
| mo | Initial Mass of Polymer |
| MRF | Materials Recovery Facilities |
| MSW | Municipal Solid Waste |
| OECD | Organisation for Economic Co-operation and Development |
| PO | Polyolefin |
| PP | Polypropylene |
| PSW | Plastic Solid Waste |
| PVC | Polyvinyl Chloride |
| SW | Solid Waste |
| T | Reaction Temperature at Desired Time (K) |
| t | Reaction time (minutes) |
| TCT | Thermo-Chemical Treatment |
| TGA | Thermogravimetric Analysis |
| Tm | Maximum Degradation Temperature (K) |
| UNGHS | United Nations Globally Harmonized System |
| α | Material Conversion |
| β | Heating Rate (°C·min−1) |
References
- Plastics—The Facts, An Analysis of European Plastics Production, Demand and Waste Data, Plastics Europe, Online Report 2017. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf (accessed on 30 July 2018).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Al-Yaqout, A.F.; Hamoda, M.F. Evaluation of landfill leachate in arid climate—A case study. Environ. Int. 2003, 29, 593–600. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Al-Nasser, A.; Al-Dhafeeri, A.T. Multi-Variable Regression Analysis for the Solid Waste Generation in The State of Kuwait. Process Saf. Environ. Prot. 2018, 119, 172–180. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management. World Bank, Urban Development and Local Government Unit of the Sustainable Development Network. Available online: https://siteresources.worldbank.org/INTURBANDEVELOPMENT/Resources/336387-1334852610766/What_a_Waste2012_Final.pdf (accessed on 30 July 2018).
- Messenger, B. Interactive Map–World’s Most Wasteful Countries, Waste Management World. 2018. Available online: https://waste-management-world.com/a/interactive-map-worlds-most-wasteful-countries. (accessed on 30 July 2018).
- Al-Jarallah, R.; Aleisa, E. A Baseline Study for Municipal Solid Waste Characterization for the State of Kuwait. Waste Manag. 2014, 34, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Al-Meshan, A.M.; Mahros, F. Recycling of municipal solid waste in the State of Kuwait. Arab. J. Sci. Eng. 2001, 26, 3–10. [Google Scholar]
- Al-Salem, S.M.; Abraham, G.; Al-Qabandi, O.A.; Dashti, A.M. Investigating the effect of accelerated weathering on the mechanical and physical properties of high content plastic solid waste (PSW) blends with virgin linear low density polyethylene (LLDPE). Polym. Test. 2015, 46, 116–121. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency, Office of Solid Waste Report (5306P). 2007 Facts and Figures, EPA530-R-08-010. November 2008. Available online: https://archive.epa.gov/epawaste/nonhaz/municipal/web/pdf/msw07-rpt.pdf (accessed on 30 July 2018).
- Department of Environment and Rural Affairs (UK). Science Directorate, Management Support and Finance Team; Research Project Final Report; Department of Environment and Rural Affairs: London, UK, 2009.
- Mersiowski, I.; Stegmann, R.; Ejlertsson, J.; Svensson, B. Long-Term Behaviour of PVC Products under Soil-Buried and Landfill Conditions; Technical University of Hamburg-Harburg: Hamburg, Germany, 1999. [Google Scholar]
- Kulikowska, D.; Klimiuk, E. The effect of landfill age on municipal leachate composition. Bioresour. Technol. 2008, 99, 5981–5985. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Shao, L.; Lü, F.; He, P. Greenhouse gas emissions during MSW landfilling in China: Influence of waste characteristics and LFG treatment measures. J. Environ. Manag. 2013, 129, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S. The Menace of Landfills in Kuwait. Middle East, Solid Waste Management. 2016. Available online: http://www.ecomena.org/landfills-kuwait/ (accessed on 30 July 2018).
- North, E.J.; Halden, R.U. Plastics and Environmental Health: The Road Ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Li, S.S. Bisphenol A and phthalates exhibit similar toxicogenomics and health effects. Gene 2012, 494, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Iacovidou, E. Closing the loop on plastic packaging materials: What is quality and how does it affect their circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef]
- He, G.; Li, J.; Zhang, F.; Wang, C.; Guo, S. Effect of multistage tensile extrusion induced fiber orientation on fracture characteristics of high density polyethylene/short glass fiber composites. Compos. Sci. Technol. 2014, 100, 1–9. [Google Scholar] [CrossRef]
- Friedrich, D.; Luible, A. Investigations on ageing of wood-plastic composites for outdoor applications: A meta-analysis using empiric data derived from diverse weathering trials. Construct. Build. Mater. 2016, 124, 1142–1152. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Bumajdad, A.; Khan, A.R.; Sharma, B.K.; Chandrasekaran, S.R.; Al-Turki, F.A.; Jassem, F.H.; Al-Dhafeeri, A.T. Non-isothermal Degradation Kinetics of Virgin Linear Low Density Polyethylene (LLDPE) and Biodegradable Polymer Blends. J. Polym. Res. 2018, 25, 111. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Sharma, B.K.; Khan, A.R.; Arnold, J.C.; Alston, S.M.; Chandrasekaran, S.R.; Al-Dhafeeri, A.T. Thermal Degradation Kinetics of Virgin Polypropylene (PP) and PP with Starch Blends Exposed to Natural Weathering. Ind. Eng. Chem. Res. 2017, 56, 5210–5220. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Jansson, A.; Möllera, K.; Hjertberg, T. Chemical degradation of a polypropylene material exposed to simulated recycling. Polym. Degrad. Stab. 2004, 84, 227–232. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Influence of natural and accelerated weathering on various formulations of linear low density polyethylene (LLDPE) films. Mater. Des. 2009, 30, 1729–1736. [Google Scholar] [CrossRef]
- Turku, I.; Kärki, T. Accelerated weathering of fire-retarded wood–polypropylene composites. Compos. Part A 2016, 81, 305–312. [Google Scholar] [CrossRef]
- Sharma, N.; Chang, L.P.; Chu, Y.L.; Ismail, H.; Ishiaku, U.S.; Mohd Ishak, Z.A. A study on the effect of pro-oxidant on the thermo-oxidative degradation behaviour of sago starch filled polyethylene. Polym. Degrad. Stab. 2001, 71, 381–393. [Google Scholar] [CrossRef]
- ASTM D 1898-68, Standard Practice for Sampling of Plastics; American Society for Testing and Materials: Philadelphia, PA, USA, 1989.
- Al-Salem, S.M.; Abraham, G.; Dashti, A.M.; Al-Qabandi, O.A. Investigating the Applicability of Mechanical Recycling in Kuwait by Studying Developed Standardized Film Samples from Virgin/Waste Polymer Resins. In Proceedings of the 15th Chemical Technologies and Chemical Engineering Conference (CHEMTECH’15), Istanbul, Turkey, 30 November–2 December 2015; pp. 278–288, ISBN 978-605-9207-17-1. [Google Scholar]
- Al-Salem, S.M.; Al-Dousari, N.M.; Abraham, G.J.; D’Souza, M.A.; Al-Qabandi, O.A.; Al-Zakri, W. Effect of Die Head Temperature (DHT) at Compounding Stage on the Degradation of Linear Low Density Polyethylene (LLDPE)/Plastic Film Waste Blends Post Accelerated Weathering. Int. J. Polym. Sci. 2016, 2016, 5147209. [Google Scholar] [CrossRef]
- ASTM D 4329, Standard Practice for Fluorescent UV Exposure of Plastics; American Society for Testing and Materials: Philadelphia, PA, USA, 2005.
- ISO 11357-1, British Standards (BS) Implementation of the International Standards Organization, Plastics–Differential Scanning Calorimetry (DSC) Part 1: General Principles; ISO: Geneva, Switzerland, 2009.
- ISO 11357-3, British Standards (BS) Implementation of the International Standards Organization, Plastics–Differential Scanning Calorimetry (DSC) Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization; ISO: Geneva, Switzerland, 2011.
- Ojeda, T.; Freitas, A.; Birck, K.; Dalmolin, E.; Jacques, R.; Bento, F.; Camargo, F. Degradability of linear polyolefins under natural weathering. Polym. Degrad. Stab. 2011, 96, 703–707. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S.; Burnhamb, A.K.; Criadoc, J.M.; Pérez-Maquedac, L.A.; Popescud, C.; Sbirrazzuolie, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P. Kinetic study of high density polyethylene (HDPE) pyrolysis. Chem. Eng. Res. Des. 2010, 88, 1599–1606. [Google Scholar] [CrossRef]
- Danon, B.; Mkhize, N.M.; van der Gryp, P.; Görgens, J.F. Combined model-free and model-based devolatilisation kinetics of tyre rubbers. Thermochim. Acta 2015, 601, 45–53. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technnol. 2012, 126, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Núñez, L.; Fraga, F.; Núñez, M.R.; Villanueva, M. Thermogravimetric study of the decomposition process of the system BADGE (n = 0)/1, 2 DCH. Polymer 2000, 41, 4635–4641. [Google Scholar]
- Al-Salem, S.M.; Khan, A.R. On the degradation kinetics of poly(ethylene terephtalate) (PET)/poly(methyl methacrylate) (PMMA) blends in dynamic thermogravimetry. Polym. Degrad. Stab. 2014, 104, 28–32. [Google Scholar] [CrossRef]
- Beg, M.D.H.; Pickering, K.L. Accelerated weathering of unbleached and bleached Kraft wood fibre reinforced polypropylene composites. Polym. Degrad. Stab. 2008, 93, 1939–1946. [Google Scholar] [CrossRef]
- Mourad, A.I. Thermo-mechanical characteristics of thermally aged polyethylene/polypropylene blends. Mater. Des. 2010, 31, 918–929. [Google Scholar] [CrossRef]
- Al-Salem, S.; Al-Hazza’a, A.; Behbehani, M.; Al-Rowaih, A.; Asiri, F.; Al-Rowaih, S.; Karam, H. Thermal and Morphological Study of Post Accelerated Weathering Virgin/Waste Polyolefin Blends; Final Report; Project Code: EM074C; KISR: Safat, Kuwait, 2018. [Google Scholar]
- Spiridon, I.; Paduraru, O.M.; Rudowski, M.; Kozlowski, M.; Darie, R.N. Assessment of changes due to accelerated weathering of low- density polyethylene/feather composites. Ind. Eng. Chem. Res. 2012, 51, 7279–7286. [Google Scholar] [CrossRef]
- Zong, R.; Wang, Z.; Liu, N.; Hu, Y.; Liao, G. Thermal Degradation Kinetics of Polyethylene and Silane-Crosslinked Polyethylene. J. App. Polym. Sci. 2005, 98, 1172–1179. [Google Scholar] [CrossRef]
- Ceamanos, J.; Mastral, J.F.; Millera, A.; Aldea, M.E. Kinetics of pyrolysis of high density polyethylene. Comparison of isothermal and dynamic experiments. J. Anal. Appl. Pyrolysis 2002, 65, 93–110. [Google Scholar] [CrossRef]
- Park, J.W.; Oh, S.C.; Lee, H.P.; Kim, H.T.; Yoo, K.O. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar] [CrossRef]
- Al-Salem, S. Mechanical and Physical Evaluation of High Content Waste/Virgin Polyolefin Blends Exposed to Natural and Accelerated Weathering; Volume I: Compounding and Engineering Products from Plastic Solid Waste (PSW); Final Report; KISR: Safat, Kuwait, 2018. [Google Scholar]
- Hafeez, S.; Manos, G.; Al-Salem, S.M.; Aristodemou, E.; Constantinou, A. Liquid fuel synthesis in microreactors: A review. React. Eng. Chem. 2018, 3, 414–432. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Influential Parameters on Natural Weathering Under Harsh Climatic Conditions of Mechanically Recycled Plastic Film Specimens. J. Environ. Mang. 2019, 230, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.A.; Pinto, F.J.; Ramos, A.M.; Gulyurtlu, I.K.; Cabrita, I.A.; Bernardo, M.S. Kinetic Evaluation of the Pyrolysis of Polyethylene Waste. Energy Fuels 2007, 21, 2489–2498. [Google Scholar] [CrossRef]










| Material Tested | Weathering/Testing Conditions | Main Findings | Reference |
|---|---|---|---|
| LDPE, LLDPE and HDPE |
|
| Gulmine et al. [27] |
| PP (un-stabilized) Recycled plastic using compression molding (7 min at 170 °C) | Elastocon heating cabinet (70 °C, air flow of 10 L·min−1) up to 8 days of exposure. | Hydroperoxides formed during aging are rapidly transformed into carbonyl groups upon compression molding. | Jansson et al. [28] |
| LLDPE (with and without hindered amine light stabilizers) | QUV following ASTM 4329 up to 50 days of continuous exposure. | Synergism of UV stabilizers and light transforming additives was noted to have an impact on stress at yield point up to 50%. | Al-Salem [29] |
| Wood/PP composites (WPC) | Xenon Test Chamber in accordance with ISO 4892-2 up to 1000 h of exposure. | α-keto carbonyl groups of cellulose increased together with vinyl groups due to PP oxidation after weathering | Turku and Karki [30] |
| Sago starch filled LLDPE composites | Dumbbell specimen in air oven at 70 °C exposed for 4 weeks. | Pro-oxidant presence manipulated carbonyl accumulation in comparison to the control samples. | Sharma et al. [31] |
| LLDPE/Waste Blends | QUV following ASTM 4329 up to 15 days of continuous exposure. | Waste content acts as a deteriorating agent in majority of blends due to weaker polymeric matrix. | Al-Salem et al. [12] |
| Unexposed Samples | Exposed Samples | |||||
|---|---|---|---|---|---|---|
| β (°C·min−1) | Ao (min−1) | Ea (kJ·min−1) | n | Ao (min−1) | Ea (kJ·min−1) | n |
| Material Code: 50/50 | ||||||
| 5 | 8.21 × 1014 | 235 | 1.1 | 4.93 × 1010 | 175 | 1.1 |
| 10 | 3.76 × 1014 | 230 | 2.65 × 1010 | 170 | ||
| 15 | 1.16 × 1014 | 225 | 1.34 × 1010 | 165 | ||
| 20 | 7.59 × 1013 | 220 | 6.65 × 109 | 160 | ||
| 25 | 3.24 × 1013 | 215 | 3.29 × 109 | 155 | ||
| Material Code: 25/75 | ||||||
| 5 | 6.06 × 1013 | 225 | 1.1 | 2.18 × 1010 | 170 | 1.1 |
| 10 | 4.26 × 1013 | 220 | 1.55 × 109 | 165 | ||
| 15 | 2.64 × 1013 | 215 | 6.14 × 109 | 160 | ||
| 20 | 1.55 × 1013 | 210 | 3.07 × 109 | 155 | ||
| 25 | 7.24 × 1012 | 205 | 1.52 × 109 | 150 | ||
| Material Code: 0/100 | ||||||
| 5 | 1.46 × 1013 | 215 | 1.1 | 9.74 × 109 | 165 | 1.1 |
| 10 | 1.48 × 1013 | 210 | 5.49 × 109 | 160 | ||
| 15 | 6.87 × 1012 | 205 | 2.82 × 109 | 155 | ||
| 20 | 3.24 × 1012 | 200 | 1.42 × 109 | 150 | ||
| 25 | 1.41 × 1012 | 195 | 7.11 × 108 | 145 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Salem, S.M.; Behbehani, M.H.; Karam, H.J.; Al-Rowaih, S.F.; Asiri, F.M. On the Kinetics of Degradation Reaction Determined Post Accelerated Weathering of Polyolefin Plastic Waste Blends. Int. J. Environ. Res. Public Health 2019, 16, 395. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16030395
Al-Salem SM, Behbehani MH, Karam HJ, Al-Rowaih SF, Asiri FM. On the Kinetics of Degradation Reaction Determined Post Accelerated Weathering of Polyolefin Plastic Waste Blends. International Journal of Environmental Research and Public Health. 2019; 16(3):395. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16030395
Chicago/Turabian StyleAl-Salem, S.M., M.H. Behbehani, H.J. Karam, S.F. Al-Rowaih, and F.M. Asiri. 2019. "On the Kinetics of Degradation Reaction Determined Post Accelerated Weathering of Polyolefin Plastic Waste Blends" International Journal of Environmental Research and Public Health 16, no. 3: 395. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16030395