Nitrogen Removal in Oligotrophic Reservoir Water by a Mixed Aerobic Denitrifying Consortium: Influencing Factors and Immobilization Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Nitrogen Removal Experiments
2.2.1. Nitrogen Removal in Oligotrophic Medium
2.2.2 Effects of Various Factors on Nitrogen Removal in Reservoir Source Water
2.2.3. Performance of Immobilized Strains in Reservoir Source Water
2.3. Analytical Methods
3. Results and Discussion
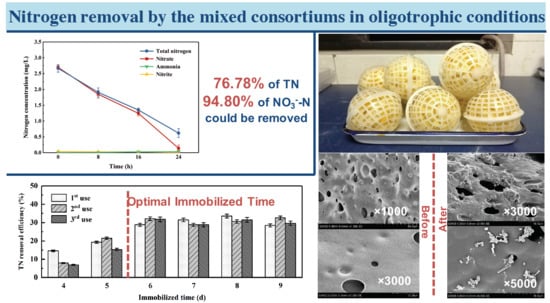
3.1. Characteristics of Nitrogen Removal in Oligotrophic Medium
3.2. Nitrogen Removal in Actual Oligotrophic Reservoir Source Water
3.2.1. Effect of Carbon Source Concentration
3.2.2. Effect of Temperature
3.2.3. Effect of Phosphorus Addition
3.3. Changes in the Consumed C/N Ratio
3.4. Nitrogen Removal Performance of Immobilized Mixed Strains in Reservoir Source Water
3.4.1. Determination of Immobilized Time
3.4.2. The Reusability of the Immobilized Strains
4. Conclusions
- The mixed aerobic denitrifying consortium used in this study had remarkable nitrogen removal effects in actual oligotrophic source water, which was limited in all types of nutrients compared to the denitrification medium, and a suitable increase in the initial TOC may be beneficial for not only the denitrification effects, but also the simultaneous removal of carbon and nitrogen.
- In the reservoir source water, a lower temperature led to slower rates of cell growth and nitrogen removal; however, its influences on overall growth and nitrogen removal effects were small. Meanwhile, the addition of phosphorus facilitated cell growth, thus improving nitrogen removal performance.
- There is a positive correlation between the initial C/N ratios and the consumed C/N ratio (ΔTOC/ΔNO3−-N and ΔTOC/ΔTN) in oligotrophic environment. For this consortium, the utilization efficiency of carbon source can be encouraged by more initial carbon source, lower temperature and phosphorus addition.
- The immobilized time of the mixed consortium on carrier balls filled with polyurethane foam cubes is about 6 days, and the ripened carriers could achieve stable denitrification ability for at least 9 cycles reuse, which verified the reusability of the immobilized consortium, providing a good reference for TN removal in practical engineering applications.
Author Contributions
Funding
Conflicts of Interest
References
- Huang, T.; Chai, B. Advances in the Study of Controlling Mechanics and Technology for Source Water Reservoir Quality. Adv. Earth Sci. 2009, 24, 588–596. [Google Scholar]
- Giannopoulos, G.; Sullivan, M.J.; Hartop, K.; Rowley, G.; Gates, A.; Watmough, N.J.; Richardson, D.J. Tuning the modular Paracoccus denitrificans respirome to adapt from aerobic respiration to anaerobic denitrification. Environ. Microbiol. 2017, 19, 4953–4964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.; Gao, N.; Yin, D.; Krasner, S.W.; Mitch, W.A. Impact of UV/H2O2 pre-oxidation on the formation of haloacetamides and other nitrogenous disinfection byproducts during chlorination. Environ. Sci. Technol. 2014, 48, 12190–12198. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, Halonitriles, Haloamides, and N-Nitrosamines: A Critical Review of Nitrogenous Disinfection Byproduct Formation Pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.B.; Gupta, S.K. Simultaneous carbon and nitrogen removal in a mixed culture aerobic RBC biofilm. Water Res. 1999, 33, 555–561. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.-Y.; Yoon, S.J.; Cho, S.J.; Kim, Y.H.; Kim, M.J.; Ryu, E.Y.; Lee, S.-J. Aerobic Denitrification of Pseudomonas putida AD-21 at Different C/N Ratios. J. Biosci. Bioeng. 2008, 106, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ni, J. Ammonium removal by Agrobacterium sp. LAD9 capable of heterotrophic nitrification–aerobic denitrification. J. Biosci. Bioeng. 2012, 113, 619–623. [Google Scholar] [CrossRef]
- Borges, M.-T.; Morais, A.; Castro, P.M.L. Performance of outdoor seawater treatment systems for recirculation in an intensive turbot (Scophthalmus maximus) farm. Aquac. Int. 2003, 11, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.D. Study on Screening Methord of Aerobic Denitrifiers. Acta Microbiol. Sin. 2004, 44, 837. [Google Scholar]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess. Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Huang, T.-L.; Zhou, S.-L.; Zhang, H.-H.; Bai, S.-Y.; He, X.-X.; Yang, X. Nitrogen Removal Characteristics of a Newly Isolated Indigenous Aerobic Denitrifier from Oligotrophic Drinking Water Reservoir, Zoogloea sp. N299. Int. J. Mol. Sci. 2015, 16, 10038–10060. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Zhang, L.; Zhang, H.; Su, J.; Guo, L.; Zhao, J.; Zhang, K. Screening and nitrogen removal characteristics of a heterotrophic nitrification-aerobic denitrification strain (in Chinese). Ecol. Environ. Sci. 2015, 37, 2681–2688. [Google Scholar]
- Kang, P.L.; Zhang, H.H.; Huang, T.L.; Chen, S.N.; Shang, P.L.; Feng, J.; Jia, J.Y. Denitrification Characteristics and Community Structure of Aerobic Denitrifiers from Lake and Reservoir Sediments. Environ. Sci. 2018, 39, 2431–2437. [Google Scholar]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; He, D.; Ma, T.; Chen, Q.; Liu, S.; Ahmad, M.; Gui, M.; Ni, J. Reducing NO and N2O emission during aerobic denitrification by newly isolated Pseudomonas stutzeri PCN-1. Bioresour. Technol. 2014, 162, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Fang, H.; Su, B.; Chen, J.; Lin, J. Characterization of a halophilic heterotrophic nitrification–aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-J.; Zhao, B.; An, Q.; Tian, M. Characteristics of a Novel Aerobic Denitrifying Bacterium, Enterobacter cloacae Strain HNR. Appl. Biochem. Biotechnol. 2016, 178, 947–959. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Zhao, B.; Cheng, D.Y.; Tan, P.; An, Q.; Guo, J.S. Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresource Technology 2018, 250, 564–573. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Zhang, D.; Qin, W. Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification–aerobic denitrification at low temperature. Bioresour. Technol. 2013, 146, 44–50. [Google Scholar] [CrossRef]
- Yang, M.; Lu, D.; Qin, B.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Highly efficient nitrogen removal of a coldness-resistant and low nutrient needed bacterium, Janthinobacterium sp. M-11. Bioresour. Technol. 2018, 256, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, D.; Li, W.; Qin, W.; Huang, X.; Lv, L. Substrates removal and growth kinetic characteristics of a heterotrophic nitrifying-aerobic denitrifying bacterium, Acinetobacter harbinensis HITLi7T at 2 °C. Bioresour. Technol. 2018, 259, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Huang, T.L.; Su, J.F.; Wang, C.Y.; Huang, Z.; Li, N. Isolation and identification of an oligotrophic and aerobic denitrification and its denitrification characteristics. Ecol. Environ. Sci. 2012, 19, 2166–2171. [Google Scholar]
- Huang, T.; Li, N.; Zhang, H.; Wang, K.; Liu, T. Denitrification characters and safety of communities of cold tolerant oligotrophic and aerobic denitrifying bacteria. Chin. J. Environ. Eng. 2013, 7, 2419–2423. [Google Scholar]
- Huang, T.; Bai, S.; Zhang, H.; Zhou, S.; He, X. Identification and denitrification characteristics of an oligotrophic heterotrophic nitrification and aerobic denitrification bacteria. Ecol. Environ. Sci. 2015, 9, 5665–5671. [Google Scholar]
- Huang, T.-L.; Zhou, S.-L.; Zhang, H.-H.; Zhou, N.; Guo, L.; Di, S.-Y.; Zhou, Z.-Z. Nitrogen removal from micro-polluted reservoir water by indigenous aerobic denitrifiers. Int. J. Mol. Sci. 2015, 16, 8008–8026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, S. Screening and Cultivation of Oligotrophic Aerobic Denitrifying Bacteria. In Water Pollution and Water Quality Control of Selected Chinese Reservoir Basins; Huang, T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 451–473. [Google Scholar]
- Joo, H.S.; Hirai, M.; Shoda, M. Improvement in ammonium removal efficiency in wastewater treatment by mixed culture of Alcaligenes faecalis no. 4 and L1. J. Biosci. Bioeng. 2007, 103, 66–73. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, T.; Bai, S.; He, X. Nitrogen removal characteristics of mixed aerobic denitrification bacteria under in-situ biological inoculation. Acta Microbiol. Sin. 2016, 56, 590–602. [Google Scholar]
- Guo, Y.; Yang, R.; Zhang, Z.; Wang, X.; Ye, X.; Chen, S. Synergy of carbon and nitrogen removal of a co-culture of two aerobic denitrifying bacterial strains, Acinetobacter sp. GA and Pseudomonas sp. GP. RSC Adv. 2018, 8, 21558–21565. [Google Scholar] [CrossRef]
- Nair, I.C.; Jayachandran, K.; Shashidhar, S. Treatment of paper factory effluent using a phenol degrading Alcaligenes sp. under free and immobilized conditions. Bioresour. Technol. 2007, 98, 714–716. [Google Scholar] [CrossRef]
- Isaka, K.; Kimura, Y.; Osaka, T.; Tsuneda, S. High-rate denitrification using polyethylene glycol gel carriers entrapping heterotrophic denitrifying bacteria. Water Res. 2012, 46, 4941–4948. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qu, Y.; Ma, F.; Zhou, J. Bioremediation of coking wastewater containing carbazole, dibenzofuran and dibenzothiphene by immobilized naphthalene-cultivated Arthrobacter sp. W1 in magnetic gellan gum. Bioresour. Technol. 2014, 166, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, A.; Cui, D.; Yang, J.; Ma, F. Performance of enhanced biological SBR process for aniline treatment by mycelial pellet as biomass carrier. Bioresour. Technol. 2011, 102, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.A.; Cui, Z.Q.; Qian, Z.Q.; Zheng, Y.H. Advances in Immobilized Microorganism and Its Applications of Wastewater Treatment. J. Chongqing Univ. 2004, 3, 125–129. [Google Scholar]
- Ma, F.; Sun, Y.; Li, A.; Zhang, X.; Yang, J. Activation of accumulated nitrite reduction by immobilized Pseudomonas stutzeri T13 during aerobic denitrification. Bioresour. Technol. 2015, 187, 30–36. [Google Scholar] [CrossRef]
- Zhou, S.-L.; Huang, T.; Bai, S.Y.; He, X.X. Isolation, Identification, and nitrogen removal characteristics of oligotrophic aerobic denitrifiers. China Environ. Sci. 2016, 36, 238–248. [Google Scholar]
- Lautenschlager, K.; Hwang, C.; Liu, W.T.; Boon, N.; Köster, O.; Vrouwenvelder, H.; Egli, T.; Hammes, F. A microbiology-based multi-parametric approach towards assessing biological stability in drinking water distribution networks. Water Res. 2013, 47, 3015. [Google Scholar] [CrossRef]
- Wen, G.; Zhu, H.; Wei, Y.; Huang, T.; Ma, J. Formation of assimilable organic carbon during the oxidation of water containing Microcystis aeruginosa by ozone and an advanced oxidation process using ozone/hydrogen peroxide. Chem. Eng. J. 2016, 307, 364–371. [Google Scholar] [CrossRef]
- Wen, G.; Kötzsch, S.; Vital, M.; Egli, T.; Ma, J. BioMig—A Method to Evaluate the Potential Release of Compounds from and the Formation of Biofilms on Polymeric Materials in Contact with Drinking Water. Environ. Sci. Technol. 2015, 49, 11659–11669. [Google Scholar] [CrossRef]
- Frederik, A.H. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 2005, 9. [Google Scholar] [CrossRef]
- Huang, T.; Xiuxiu, H.E.; Zhang, H.; Zhou, S.; Bai, S. Nitrogen removal characteristics of the heterotrophic nitrification-aerobic denitrification bacterium Acinetobacter sp. Sxf14. Chin. J. Appl. Environ. Biol. 2015, 21, 201–207. [Google Scholar]
- Chen, J.; Gu, S.; Hao, H.; Chen, J. Characteristics and metabolic pathway of Alcaligenes sp. TB for simultaneous heterotrophic nitrification-aerobic denitrification. Appl. Microbiol. Biotechnol. 2016, 100, 9787–9794. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Rittmann, B.E. Effects of pH and precipitation on autohydrogenotrophic denitrification using the hollow-fiber membrane-biofilm reactor. Water Res. 2003, 37, 1551–1556. [Google Scholar] [CrossRef]
- Wang, Z.; He, S.; Huang, J.; Zhou, W.; Chen, W. Comparison of heterotrophic and autotrophic denitrification processes for nitrate removal from phosphorus-limited surface water. Environ. Pollut. 2018, 238, 562–572. [Google Scholar] [CrossRef]









| Index | TN (mg/L) | NO3−-N (mg/L) | NO2−-N (mg/L) | NH4+-N (mg/L) | TP (mg/L) | TOC (mg/L) | CODMn (mg/L) |
| Value | 2.64 ± 0.23 | 2.59 ± 0.47 | 0.01 ± 0.01 | 0.20 ± 0.08 | 0.22 ± 0.06 | 2.45 ± 0.36 | 5.11 ± 0.48 |
| Index | Fe (mg/L) | Mn (mg/L) | pH | ORP (mV) | Conductivity (μS/cm) | Turbidity (NTU) | DO (mg/L) |
| Value | 0.16 ± 0.06 | 0.02 ± 0.02 | 7.60 ± 0.15 | 186 ± 11 | 200 ± 2 | 32.45 ± 2.74 | 8.49 ± 0.48 |
| Experimental Conditions | Initial | Final | Removal Efficiency (%) | ||||
|---|---|---|---|---|---|---|---|
| C/N (mg/mg) | ΔTOC/ΔNO3−-N (mg/mg) | ΔTOC/ΔTN (mg/mg) | NO3−-N | TN | TOC | ||
| Denitrification medium | 8.00 | 8.02 | 10.03 | 94.76 | 75.83 | 95.00 | |
| Source water | 5 mg/L TOC | 2.64 | 3.19 | 3.09 | 35.87 | 33.47 | 43.24 |
| 10 mg/L TOC | 5.62 | 10.31 | 9.41 | 41.59 | 39.84 | 76.37 | |
| 15 mg/L TOC | 7.96 | 10.50 | 10.85 | 64.44 | 57.79 | 84.98 | |
| 20 mg/L TOC | 9.58 | 10.71 | 12.37 | 75.32 | 63.11 | 84.20 | |
| 20 °C / Without P | 8.61 | 17.09 | 17.89 | 47.52 | 50.39 | 94.01 | |
| 10 °C / Without P | 9.25 | 11.04 | 15.33 | 63.17 | 48.64 | 79.84 | |
| 20 °C / With P | 5.11 | 6.27 | 6.77 | 68.92 | 68.08 | 84.60 | |
| HITLi7T Denitrification medium/2 °C | 5.30 | 4.13 | - | 7.78 | - | 5.89 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, T.; Yang, S.; Liu, X.; Kou, L.; Huang, T.; Wen, G. Nitrogen Removal in Oligotrophic Reservoir Water by a Mixed Aerobic Denitrifying Consortium: Influencing Factors and Immobilization Effects. Int. J. Environ. Res. Public Health 2019, 16, 583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16040583
Wang H, Wang T, Yang S, Liu X, Kou L, Huang T, Wen G. Nitrogen Removal in Oligotrophic Reservoir Water by a Mixed Aerobic Denitrifying Consortium: Influencing Factors and Immobilization Effects. International Journal of Environmental Research and Public Health. 2019; 16(4):583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16040583
Chicago/Turabian StyleWang, Hanyue, Tong Wang, Shangye Yang, Xueqing Liu, Liqing Kou, Tinglin Huang, and Gang Wen. 2019. "Nitrogen Removal in Oligotrophic Reservoir Water by a Mixed Aerobic Denitrifying Consortium: Influencing Factors and Immobilization Effects" International Journal of Environmental Research and Public Health 16, no. 4: 583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16040583