Dredging Activities Carried Out in a Brazilian Estuary Affect Mercury Levels in Swimming Crabs
Abstract
:1. Introduction
2. Materials and Methods
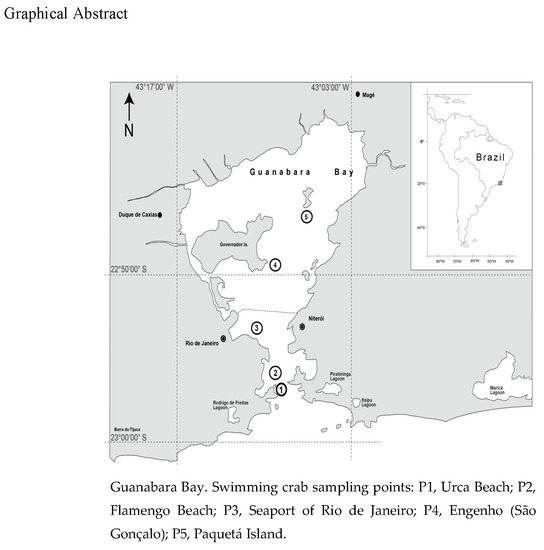
2.1. Study Area
2.2. Swimming Crab and Water Samplings
2.3. Biometric Swimming Crab Processing
2.4. Mercury Analysis
2.4.1. Sample Analysis
2.4.2. Calibration Curve
2.4.3. Optimization of Analytical Conditions
2.4.4. Human Consumption Risks
2.5. Statistical Analyses
3. Results
3.1. Abiotic Variables
3.2. Swimming Crab THg Concentrations and Correlations to Abiotic Factors
4. Discussion
4.1. Abiotic Variables
4.2. Factors Affecting Hg Concentrations
4.3. Risk to Consumption
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baptista Neto, J.A.; Barreto, C.F.; Vilela, C.G.; Fonseca, E.M.; Melo, G.V.; Barth, O.M. Environmental change in Guanabara Bay, SE Brazil, based in microfauna, pollen and geochemical proxies in sedimentary cores. Ocean Coast. Manag. 2016, 143, 4–15. [Google Scholar] [CrossRef]
- Kehrig, H.A.; Malm, O.; Palermo, E.F.A.; Seixas, T.G.; Baêta, A.P.; Moreira, I. Bioconcentração e biomagnificação de metilmercúrio na baía de Guanabara, Rio de Janeiro. Química Nova 2011, 34, 377–384. [Google Scholar] [CrossRef]
- Barletta, M.; Lima, A.R.A.; Costa, M.F. Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries. Sci. Total Environ. 2019, 651, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Soares-Gomes, A.; Da Gama, B.A.P.; Baptista Neto, J.A.; Freire, D.G.; Cordeiro, R.C.; Machado, W.; Bernardes, M.C.; Coutinho, R.; Thompson, F.; Pereira, R.C. An environmental overview of Guanabara Bay, Rio de Janeiro. Reg. Stud. Mar. Sci. 2016, 8, 319–330. [Google Scholar] [CrossRef]
- Rodrigues, P.A.; Ferrari, R.G.; Hauser-Davis, R.A.; Santos, L.N.; Conte-Junior, C.A. Seasonal influences on swimming crab mercury levels in an eutrophic estuary located in southeastern Brazil. Environ. Sci. Pollut. Res. 2019, 27, 3473–3482. [Google Scholar] [CrossRef]
- Taylor, D.L.; Calabrese, N.M. Mercury content of blue crabs (Callinectes sapidus) from southern New England coastal habitats: Contamination in an emergent fishery and risks to human consumers. Mar. Pollut. Bull. 2018, 126, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Ruus, A.; Hjermann, D.O.; Beylich, B.; Schoyen, M.; Oxnevad, S.; Green, N.W. Mercury concentration trend as a possible result of changes in cod population demography. Mar. Environ. Res. 2017, 130, 85–92. [Google Scholar] [CrossRef]
- Roman, H.A.; Walsh, T.L.; Coull, B.A.; Dewailly, E.; Guallar, E.; Hattis, D.; Marien, K.; Schwartz, J.; Stern, A.H.; Virtanen, J.K.; et al. Evaluation of the Cardiovascular Effects of Methylmercury Exposures: Current Evidence Supports Development of a Dose–Response Function for Regulatory Benefits Analysis. Environ. Health Perspect. 2011, 119, 607–614. [Google Scholar] [CrossRef]
- Owens, E.; Effer, S.W.; Bookman, R.; Driscoll, C.T.; Matthews, D.A.; Effier, A.J.P. Resuspension of Mercury-Contaminated Sediments from an In-Lake Industrial Waste Deposit. J. Environ. Eng. 2009, 135, 526–534. [Google Scholar] [CrossRef]
- Fistarol, G.O.; Coutinho, F.H.; Moreira, A.P.B.; Venas, T.; Cánovas, A.; Paula, S.E.M., Jr.; Coutinho, R.; Moura, R.L.; Valentin, J.L.; Thompson, F.L.; et al. Environmental and Sanitary Conditions of Guanabara Bay, Rio de Janeiro. Front. Microbiol. 2015, 6, 1232. [Google Scholar] [CrossRef] [Green Version]
- Silveira, A.E.F.; Nascimento, J.R.; Sabadini-Santos, E.; Bidone, E.D. Screening-level risk assessment applied to dredging of polluted sediments from Guanabara Bay, Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2017, 118, 368–375. [Google Scholar] [CrossRef]
- Chen, M.M.; Lopez, L.; Bhavsar, S.P.; Sharma, S. What’s hot about mercury? Examining the influence of climate on mercury levels in Ontario top predator fishes. Environ. Res. 2018, 162, 63–73. [Google Scholar] [PubMed]
- Seixas, L.B.; Santos, L.N.; Santos, A.F.G.N. Fluctuating asymmetry: A tool for impact assessment on fish populations in a tropical polluted bay, Brazil. Ecol. Indicat. 2016, 71, 522–532. [Google Scholar] [CrossRef]
- Andrade, L.S.; Frameschi, I.F.; Costa, R.C.; Castilho, A.L.; Fransozo, A. The assemblage composition and structure of swimming crabs (Portunoidea) in continental shelf waters of southeastern Brazil. Cont. Shelf Res. 2014, 94, 8–16. [Google Scholar] [CrossRef]
- Arcagni, M.; Juncos, R.; Rizzo, A.; Pavlin, M.; Fajon, V.; Arribére, M.A.; Horvat, M.; Ribeiro Guevara, S. Species- and habitat-specific bioaccumulation of total mercury and methylmercury in the food web of a deep oligotrophic lake. Sci. Total Environ. 2018, 612, 1311–1319. [Google Scholar] [CrossRef]
- Bisi, T.L.; Lepoint, G.; Azevedo, A.D.F.; Dorneles, P.R.; Flach, L.; Das, K.; Malm, O.; Lailson-brito, J. Trophic relationships and mercury biomagnification in Brazilian tropical coastal food webs. Ecol. Indic. 2012, 18, 291–302. [Google Scholar] [CrossRef]
- Van Hees, K.E.; Ebert, D.A. An evaluation of mercury offloading in two Central California elasmobranchs. Sci. Total Environ. 2017, 590, 154–162. [Google Scholar] [CrossRef]
- Rasinger, J.D.; Lundebye, A.K.; Penglase, S.J.; Ellingsen, S.; Amlund, H. Methylmercury Induced Neurotoxicity and the Influence of Selenium in the Brains of Adult Zebrafish (Danio rerio). Int. J. Mol. Sci. 2017, 18, 725. [Google Scholar] [CrossRef]
- Barroso, R.M.; Wiefels, A.C. O Mercado Pesqueiro da Região Metropolitana do Rio de Janeiro. 2010. Available online: http://www2.produce.gob.pe/RepositorioAPS/3/jer/DGA-PUBLICACIONES/Mercado-de-Pescado-en-Rio-de-Janeiro.pdf (accessed on 14 December 2019).
- Genç, T.O.; Yulmaz, F. Metal Accumulations in Water, Sediment, Crab (Callinectes sapidus) and Two Fish Species (Mugil cephalus and Anguilla anguilla) from the Köyceğiz Lagoon System-Turkey: An Index Analysis Approach. Bull. Environ. Contam. Toxicol. 2017, 99, 173–181. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Johnson-Restrepo, B.; Baldiris-Avila, R.; GuetteFernandez, J.; Magallanes-Carreazo, E.; Venegas-Ramírez, L.; Kunihiko, N. Human and crab exposure to mercury in the Caribbean coastal shoreline of Colombia: Impact from an abandoned chlor-alkali plant. Environ. Int. 2008, 34, 476–482. [Google Scholar] [CrossRef]
- Moura, V.L.; Lacerda, L.D. Contrasting Mercury Bioavailability in the Marine and Fluvial Dominated Areas of the Jaguaribe River Basin, Ceará, Brazil. Bull. Environ. Contam. Toxicol. 2018, 101, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Linehan, J.C.; Murray, D.W.; Prell, W.L. Indicators of sediment and biotic mercury contamination in a southern New England estuary. Mar. Pollut. Bull. 2012, 64, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Reichmuth, J.M.; Weis, P.; Weis, J.S. Bioaccumulation and depuration of metals in blue crabs (Callinectes sapidus Rathbun) from a contaminated and clean estuary. Environ. Pollut. 2010, 158, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Bordon, I.C.A.C.; Sarkis, J.E.S.; Toma’s, A.R.; Scalco, A.; Lima, M.; Hortellani, M.A.; Andrade, N.P. Assessment of metal concentrations in muscles of the blue crab, Callinectes danae S, from the Santos Estuarine System. Bull. Environ. Contam. Toxicol. 2012, 89, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Bordon, I.C.A.C.; Sarkis, J.E.S.; Andrade, N.P.; Hortellani, M.A.; Favaro, D.I.T.; Kakazu, M.H.; Cotrim, M.E.B.; Lavradas, R.T.; Moreira, I.; Saint’Pierre, T.D.; et al. An environmental forensic approach for tropical estuaries based on metal bioaccumulation in tissues of Callinectes danae. Ecotoxicology 2016, 25, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.C.N.R.; Franco, A.C.S.; Seixas, L.B.; Cruz, L.R.C.; Santos, L.N. Testing the ecocline concept for fish assemblages along the marine-estuarine gradient in a highly-eutrophic estuary (Guanabara Bay, Brazil). Estuar. Coast. Shelf Sci. 2018, 12, 118–126. [Google Scholar] [CrossRef]
- Barreto, N.R.; Brotto, D.V.S.; Giordano, R.G.; Bertoncini, A.A.; Santos, L.N. The rocky reef fishes of Vermelha Beach, a marine estuarine transitional zone at Guanabara Bay, Brazil. Latin Am. J. Aquat. Res. 2017, 45, 33–40. [Google Scholar] [CrossRef]
- Crab Database, Taxon Tree of Crabs. 2016. Available online: https://www.crabdatabase.info/en/crabs (accessed on 12 December 2019).
- Food and Agriculture Organization of the United Nations. FAO FishFinder. Species Fact Sheets. 2018. Available online: http://www.fao.org/fishery/species/2632/en (accessed on 12 December 2019).
- Guimarães, C.F.M.; Mársico, E.T.; Monteiro, M.L.G.; Lemos, M.; Mano, S.B.; Conte Junior, C.A. The chemical quality of frozen Vietnamese Pangasius hypophthalmus fillets. Food Sci. Nutr. 2016, 4, 389–408. [Google Scholar] [CrossRef]
- Torres, D.P.; Martins-Teixeira, M.B.; Silva, E.F.; Queiroz, H.M. Method development for the control determination of mercury in seafood by solid-sampling thermal decomposition amalgamation atomic absorption spectrometry (TDA AAS). Food Addit. Contam. Part A 2012, 29, 625–632. [Google Scholar] [CrossRef]
- Fries, A.; Coimbra, J.P.; Nemazie, D.A.; Summers, R.M.; Azevedo, J.P.S.; Filoso, S.; Newton, M.; Gelli, G.; Oliveira, R.C.N.; Pessoa, M.A.R.; et al. Guanabara Bay ecosystem health report card: Science, management, and governance implications. Reg. Stud. Mar. Sci. 2019, 25, 100474. [Google Scholar] [CrossRef]
- Souza, J.S.; Santos, L.N.; Santos, A.F.G.N. Habitat features not water variables explain most of fish assemblages use of sandy beaches in a Brazilian eutrophic bay. Estuar. Coast. Shelf Sci. 2018, 7, 100–109. [Google Scholar] [CrossRef]
- Choppala, G.; Moon, E.; Bush, R.; Bolan, N.; Carroll, N. Dissolution and redistribution of trace elements and nutrients during dredging of iron monosulfide enriched sediments. Chemosphere 2018, 201, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Manullang, C.Y.; Hutabarat, J.; Widowati, I. Bioaccumulation of cadmium(Cd) by white shrimp Penaeus merguiensis at diferents salinity in Kedungmalang estuary, Jepara (Central Java). Mar. Res. Indones. 2014, 39, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Sadhu, A.K.; Kim, J.P.; Furrell, H.; Bostock, B. Methyl mercury concentrations in edible fish and shellfish from Dunedin, and other regions around the South Island, New Zealand. Mar. Pollut. Bull. 2015, 101, 386–390. [Google Scholar] [CrossRef]
- Mallory, M.L.; O’Driscoll, N.J.; Klapstein, S.; Varela, J.L.; Ceapa, C.; Stokesbury, M.J. Methylmercury in tissues of Atlantic sturgeon (Acipenser oxyrhynchus) from the Saint John River, New Brunswick, Canada. Mar. Pollut. Bull. 2018, 126, 250–254. [Google Scholar] [CrossRef]
- Souza-Araujo, J.; Giarrizzo, T.; Lima, M.O.; Souza, M.B. Mercury and methyl mercury in fishes from Bacajá River (Brazilian Amazon): Evidence for bioaccumulation and biomagnification. J. Fish Biol. 2016, 89, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Viera, T.C.; Rodrigyes, A.P.C.; Amaral, P.M.G.; Oliveira, D.F.C.; Gonçalves, R.A.; Silva, C.R.; Vasques, R.O.; Malm, O.; Silva-Filho, E.V.; Godoy, J.M.O.; et al. Evaluation of the bioaccumulation kinetics of toxic metals in fish (A. brasiliensis) and its application on monitoring of coastal ecosystems. Mar. Pollut. Bull. 2020, 151, 110830. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Wei, L.; Jingfeng, Y.; Chernick, M.; Hinton, D.E. Developmental toxicity from exposure to various forms of mercury compounds in medaka fish (Oryzias latipes) embryos. Peer J. 2016, 4, e2282. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, B.L.; Kidd, K.A.; Curry, R.A.; O’driscoll, N.J.; Pavey, S.A. Mercury bioaccumulation in aquatic biota along a salinity gradient in the Saint John River estuary. J. Environ. Sci. 2018, 68, 41–54. [Google Scholar] [CrossRef]
- Karar, S.; Hazra, S.; Das, S. Assessment of the heavy metal accumulation in the Blue Swimmer Crab (Portunuspelagicus), northern Bay of Bengal: Role of salinity. Mar. Pollut. Bull. 2019, 143, 101–108. [Google Scholar] [CrossRef]
- Lee, J.A.; Marsden, I.D.; Glover, C.N. The influence of salinity on copper accumulation and its toxic effects in estuarine animals with differing osmoregulatory strategies. Aquat. Toxicol. 2010, 99, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.S.; Yu, R.Q.; Barkay, T.; Hamilton, T.L.; Baxter, B.K.; Naftaz, D.L.; Marvin-Dipasquale, M. Effect of salinity on mercury methylating benthic microbesand their activities in Great Salt Lake, Utah. Sci. Total Environ. 2017, 581, 495–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, A.; MacFarlane, G.R.; Nowak, B.; Moltschaniwskyj, N.A.; Taylor, M.D. Lethal and Sub-Lethal Effects of Aluminium on a Juvenile Penaeid Shrimp. Thalass. Int. J. Mar. Sci. 2019, 35, 359–368. [Google Scholar] [CrossRef]
- European Union (EU). Commission Regulation (EC) No 1881/2006; Official Journal of the European Union: Brussels, Belgium, 19 December 2006. [Google Scholar]
- ANVISA, Ministério da Saúde, Agência Nacional de Vigilância SanitE1ria. RDC n 42 de 29 de agosto de 2013. Dispõe sobre o Regulamento Técnico MERCOSUL sobre Limites Máximos de Contaminantes Inorgánicos em Alimentos; Diário Oficial [da] República Federativa do Brasil: Brasília, Brazil, 29 August 2013.
- Raknuzzaman, M.; Ahmed, M.K.; Islam, M.S.; Habibullahalmamun; Tokumura, M.; Sekine, M.; Masunaga, S. Trace metal contamination in commercial fish and crustaceans collected from coastal area of Bangladesh and health risk assessment. Environ. Sci. Pollut. Res. 2016, 23, 17298–17310. [Google Scholar] [CrossRef]
- JECFA/WHO. Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives. 2011. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1806 (accessed on 12 December 2019).
- National Research Council. Toxicology Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000.





| Variable | Area | Period | ||
|---|---|---|---|---|
| Before | During | After | ||
| Depth (m) | Urca | 9.00 ± 1.41 | 7.50 ± 2.47 | 7.50 ± 2.47 |
| Flamengo | 11.00 ± 4.24 | 10.60 ± 0.84 | 10.60 ± 0.84 | |
| Seaport | 7.00 ± 0.00 | 6.50 ± 0.00 | 6.50 ± 0.00 | |
| Engenho | 10.75 ± 5.30 | 8.25 ± 0.35 | 8.25 ± 0.35 | |
| Paquetá | 15.00 ± 2.82 | 16.50 ± 6.36 | 16.50 ± 6.36 | |
| Transparency (m) | Urca | 1.25 ± 0.07 | 0.85 ± 0.07 | 4.45 ± 2.19 |
| Flamengo | 0.85 ± 0.21 | 1.55 ± 0.07 | 4.25 ± 0.35 | |
| Seaport | 0.90 ± 0.14 | 1.05 ± 0.32 | 1.05 ± 0.07 | |
| Engenho | 0.85 ± 0.07 | 0.30 ± 0.14 | 0.80 ± 0.00 | |
| Paquetá | 1.00 ± 0.00 | 0.58 ± 0.10 | 0.80 ± 0.00 | |
| pH | Urca | 8.49 ± 0.03 | 8.30 ± 0.08 | 8.51 ± 0.21 |
| Flamengo | 8.75 ± 0.01 | 8.16 ± 0.12 | 8.48 ± 0.13 | |
| Seaport | 8.58 ± 0.06 | 8.01 ± 0.09 | 8.45 ± 0.15 | |
| Engenho | 8.49 ± 0.03 | 7.95 ± 0.50 | 8.36 ± 0.15 | |
| Paquetá | 8.05 ± 0.27 | 7.98 ± 0.06 | 8.67 ± 0.13 | |
| Oxygen (mg L−1) | Urca | 5.26 ± 0.50 | 9.84 ± 0.29 | 5.99 ± 0.50 |
| Flamengo | 5.74 ± 0.25 | 8.41 ± 0.17 | 6.80 ± 1.93 | |
| Seaport | 4.24 ± 0.03 | 6.07 ± 0.24 | 4.36 ± 0.73 | |
| Engenho | 3.20 ± 0.97 | 4.64 ± 0.22 | 4.05 ± 0.08 | |
| Paquetá | 3.18 ± 0.12 | 8.54 ± 3.72 | 3.07 ± 1.05 | |
| Salinity (g L−1) | Urca | 32.67 ± 0.59 | 31.72 ± 0.16 | 28.97 ± 0.36 |
| Flamengo | 32.80 ± 0.10 | 32.54 ± 0.35 | 29.79 ± 0.10 | |
| Seaport | 31.17 ± 0.55 | 31.86 ± 0.25 | 28.38 ± 0.39 | |
| Engenho | 31.72 ± 1.39 | 31.81 ± 0.29 | 29.14 ± 0.33 | |
| Paquetá | 32.66 ± 0.02 | 32.10 ± 0.41 | 28.60 ± 0.84 | |
| Temperature (°C) | Urca | 23.76 ± 0.55 | 25.32 ± 0.007 | 24.96 ± 0.31 |
| Flamengo | 23.96 ± 0.23 | 25.18 ± 0.14 | 24.77 ± 0.06 | |
| Seaport | 24.88 ± 0.36 | 25.57 ± 0.10 | 24.25 ± 0.46 | |
| Engenho | 23.91 ± 1.01 | 24.81 ± 0.10 | 23.50 ± 0.24 | |
| Paquetá | 23.00 ± 0.06 | 24.87 ± 0.13 | 23.93 ± 1.11 | |
| Collect Points | THg before Dredging | THg during Dredging | THg after Dredging |
|---|---|---|---|
| Internal Points (Engenho and Paquetá) | 0.052 ± 0.020 | 0.111 ± 0.052 | 0.108 ± 0.008 |
| Seaport | 0.098 ± 0.114 | - | 0.111 ± 0.069 |
| External Points (Urca and Flamengo) | 0.097 ± 0.004 | 0.155 ± 0.06 | 0.110 ± 0.025 |
| Location | 2016 | 2017 | 2018 |
|---|---|---|---|
| Internal areas | 0.034 | 0.055 | 0.039 |
| Port | 0.035 | - | 0.040 |
| External areas | 0.018 | 0.039 | 0.038 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.d.A.; Ferrari, R.G.; Hauser-Davis, R.A.; Neves dos Santos, L.; Conte-Junior, C.A. Dredging Activities Carried Out in a Brazilian Estuary Affect Mercury Levels in Swimming Crabs. Int. J. Environ. Res. Public Health 2020, 17, 4396. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17124396
Rodrigues PdA, Ferrari RG, Hauser-Davis RA, Neves dos Santos L, Conte-Junior CA. Dredging Activities Carried Out in a Brazilian Estuary Affect Mercury Levels in Swimming Crabs. International Journal of Environmental Research and Public Health. 2020; 17(12):4396. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17124396
Chicago/Turabian StyleRodrigues, Paloma de Almeida, Rafaela Gomes Ferrari, Rachel Ann Hauser-Davis, Luciano Neves dos Santos, and Carlos Adam Conte-Junior. 2020. "Dredging Activities Carried Out in a Brazilian Estuary Affect Mercury Levels in Swimming Crabs" International Journal of Environmental Research and Public Health 17, no. 12: 4396. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17124396