Usability Assessment of an Innovative Device in Infusion Therapy: A Mix-Method Approach Study
Abstract
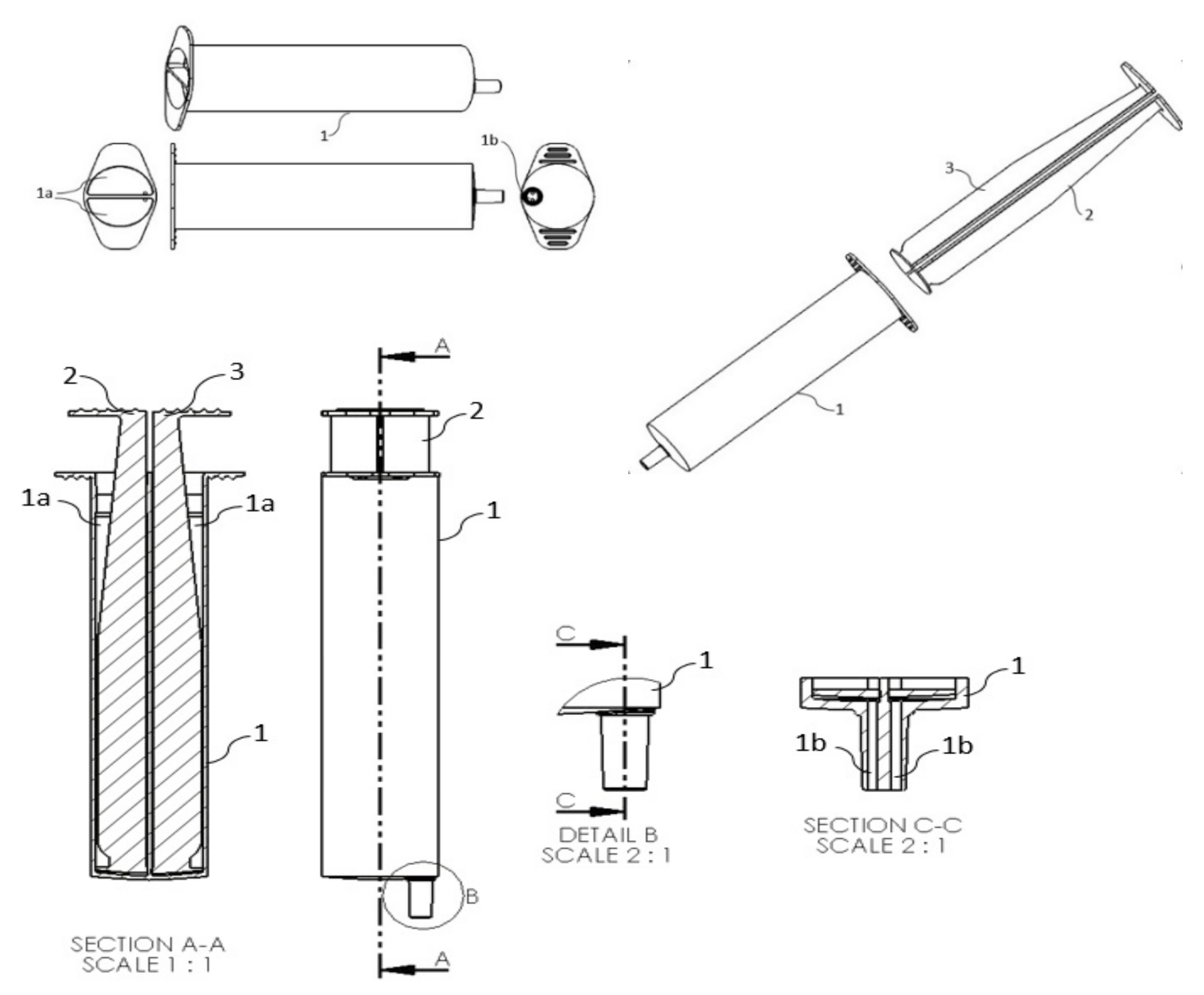
:1. Introduction
2. Materials and Methods
2.1. Design and Procedures
2.2. Sample
2.3. Usability Questionnaire
2.4. Ethics
2.5. Data Analysis
3. Results
3.1. Usability Questionnaire Development and Pilot Study
3.2. Double-Chamber Syringe Usability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carr, P.J.; Higgins, N.S.; Cooke, M.L.; Mihala, G.; Rickard, C.M. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Database Syst. Rev. 2018, 3, CD011429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Rickard, C.; Rickard, C.; Domer, G.S.; Kaur, P. Dangers of peripheral intravenous catheterization: The forgotten tourniquet and other patient safety considerations. Intechopen 2019. [Google Scholar] [CrossRef] [Green Version]
- Alexandrou, E.; Ray-Barruel, G.; Carr, P.J.; Frost, S.; Inwood, S.; Higgins, N.; Lin, F.; Alberto, L.; Mermel, L.; Rickard, C.M. International prevalence of the use of peripheral intravenous catheters. J. Hosp. Med. 2015, 10, 530–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattox, E.A. Complications of peripheral venous access devices: Prevention, detection, and recovery strategies. Crit. Care Nurse 2017, 37, e1–e14. [Google Scholar] [CrossRef] [Green Version]
- Miliani, K.; Taravella, R.; Thillard, D.; Chauvin, V.; Martin, E.; Edouard, S.; Astagneau, P.; CATHEVAL Study Group. Peripheral venous catheter-related adverse events: Evaluation from a multicentre epidemiological study in France (the CATHEVAL project). PLoS ONE 2017, 12, e0168637. [Google Scholar] [CrossRef]
- Abolfotouh, M.A.; Salam, M.; Bani-Mustafa, A.; White, D.; Balkhy, H.H. Prospective study of incidence and predictors of peripheral intravenous catheter-induced complications. Ther. Clin. Risk Manag. 2014, 10, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Cicolini, G.; Manzoli, L.; Simonetti, V.; Flacco, M.E.; Comparcini, D.; Capasso, L.; Di Baldassarre, A.; Eltaji Elfarouki, G. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: A large multi-centre prospective study. J. Adv. Nurs. 2014, 70, 2539–2549. [Google Scholar] [CrossRef]
- Dunda, S.E.; Demir, E.; Mefful, O.J.; Grieb, G.; Bozkurt, A.; Pallua, N. Management, clinical outcomes, and complications of acute cannula-related peripheral vein phlebitis of the upper extremity: A retrospective study. Phlebology 2015, 30, 381–388. [Google Scholar] [CrossRef]
- Oliveira, A.S.S.; Parreira, P.M.S.D. Intervenções de enfermagem e flebites decorrentes de cateteres venosos periféricos. Revisão sistemática da literatura. Rev. Enferm. Ref. 2010, serIII, 137–147. [Google Scholar] [CrossRef]
- Danski, R.; Tannia, M.; Johann, A.; Vayego, A.; Paulo, S. Artigo Original Complicações relacionadas ao uso do cateter venoso periférico: Ensaio clínico randomizado. Acta Paul Enferm. 2016, 29, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Arvaniti, K.; Lathyris, D.; Blot, S.; Apostolidou-Kiouti, F.; Koulenti, D.; Haidich, A.B. Cumulative Evidence of Randomized Controlled and Observational Studies on Catheter-Related Infection Risk of Central Venous Catheter Insertion Site in ICU Patients. Crit. Care Med. 2017, 45, e437–e448. [Google Scholar] [CrossRef] [PubMed]
- Beloin, C.; Fernandez-Hidalgo, N.; Lebeaux, D. Understanding biofilm formation in intravascular device-related infections. Intensive Care Med. 2017, 43, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guembe, M.; Pérez-Granda, M.J.; Capdevila, J.A.; Barberán, J.; Pinilla, B.; Martín-Rabadán, P.; Bouza, E.; NUVE Study Group. Nationwide study on peripheral-venous-catheter-associated-bloodstream infections in internal medicine departments. J. Hosp. Infect. 2017, 97, 260–266. [Google Scholar] [CrossRef]
- Keogh, S.; Flynn, J.; Marsh, N.; Higgins, N.; Davies, K.; Rickard, C.M. Nursing and midwifery practice for maintenance of vascular access device patency. A cross-sectional survey. Int. J. Nurs. Stud. 2015, 52, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorski, L.A.; Hadaway, L.; Hagle, M.; McGoldrick, M.; Orr, M.; Doellman, D. The 2016 Infusion Therapy Standards of Practice; Wolters Kluwer: South Holland, The Netherlands, 2016. [Google Scholar]
- Queensland Government, Department of Health. Guidelines: Peripheral Intravenous Catheter (PIVC); Queensland Government: Queensland, Australia, 2018; pp. 1–28. [Google Scholar]
- Royal College of Nursing (Andrea Denton). Standards for Infusion Therapy; R Coll Nurs; Royal College of London: London, UK, 2016; pp. 41–42. [Google Scholar]
- Parreira, P.; Sousa, L.B.; Marques, I.A.; Costa, P.; Cortez, S.; Carneiro, F.; Cruz, A.; Salgueiro-Oliveira, A. Development of an innovative double-chamber syringe for intravenous therapeutics and flushing: Nurses’ involvement through a human-centred approach. PLoS ONE 2020, 15, e0235087. [Google Scholar] [CrossRef]
- Ministério da Saúde. Decreto-Lei n.o 145/2009 de 17 de Junho (Regras a que Devem Obedecer a Investigação, o Fabrico, a Comercialização, a Entrada em Serviço, a Vigilância e a Publicidade dos Dispositivos Médicos e Respectivos Acessórios); Ministério da Saúde: Lisbon, Portugal, 2009. [Google Scholar]
- European Council’s Recent Revision in MedDev 2. 7.1 Guidelines (Revision 4)—Quantifying its Change and its Impact. J. Bioeng. Biomed. Sci. 2017, 7, 1–7. [Google Scholar]
- Legislation117. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EE. Off. J. Eur. Union 2017, 60, 2–175.
- Privitera, M.B.; Evans, M.; Southee, D. Human factors in the design of medical devices – Approaches to meeting international standards in the European Union and USA. Appl. Ergon. 2017, 59, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Vincent, C.J.; Li, Y.; Blandford, A. Integration of human factors and ergonomics during medical device design and development: It’s all about communication. Appl. Ergon. 2014, 45, 413–419. [Google Scholar] [CrossRef]
- Ciurana, J. Designing, prototyping and manufacturing medical devices: An overview. Int. J. Comput. Integr. Manuf. 2014, 27, 901–918. [Google Scholar] [CrossRef]
- Tarricone, R.; Torbica, A.; Drummond, M. Challenges in the Assessment of Medical Devices: The MedtecHTA Project. Health Econ. UK 2017, 26, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciani, O.; Wilcher, B.; van Giessen, A.; Taylor, R.S. Linking the Regulatory and Reimbursement Processes for Medical Devices: The Need for Integrated Assessments. Health Econ. 2017, 26, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, S.; Olberg, B.; Panteli, D.; Perleth, M.; Busse, R. HTA of medical devices: Challenges and ideas for the future from a European perspective. Health Policy 2017, 121, 215–229. [Google Scholar] [CrossRef] [PubMed]
- EUnetHTA Joint Action 2, Work Package 8. HTA Core Model ® version 3.0 (Pdf). EUnetHTA JA; National Institute for Health and Welfare: Helsinki, Finland, 2016; Volume 2, pp. 1–410.
- Harte, R.; Glynn, L.; Rodríguez-Molinero, A.; Baker, P.M.; Scharf, T.; Quinlan, L.R.; ÓLaighin, G. A Human-Centered Design Methodology to Enhance the Usability, Human Factors, and User Experience of Connected Health Systems: A Three-Phase Methodology. JMIR Hum. Factors 2017, 4, e8. [Google Scholar] [CrossRef]
- ISO 9241-11: 2018. Ergonomics of Human-System Interaction—Part 11: Usability: Definitions and Concepts; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Kortum, P.T.; Bangor, A. Usability Ratings for Everyday Products Measured With the System Usability Scale. Int. J. Hum. Comput. Interact. 2013, 29, 67–76. [Google Scholar] [CrossRef]
- Johnson, C.M.; Johnson, T.R.; Zhang, J. A user-centered framework for redesigning health care interfaces. J. Biomed. Inform. 2005, 38, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Wiklund, M.; Kendler, J.; Strochlic, A. Usability Testing of Medical Devices, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4665-9588-0. [Google Scholar]
- Money, A.G.; Barnett, J.; Kuljis, J.; Craven, M.P.; Martin, J.L.; Young, T. The role of the user within the medical device design and development process: Medical device manufacturers’ perspectives. BMC Med. Inform. Decis. Mak. 2011, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Murphy, E.; Crowe, J.A.; Norris, B.J. Capturing user requirements in medical device development: The role of ergonomics. Physiol. Meas. 2006, 27, R49–R62. [Google Scholar] [CrossRef]
- Borsci, S.; Macredie, R.D.; Martin, J.L.; Young, T. How many testers are needed to assure the usability of medical devices? Expert Rev. Med. Devices 2014, 11, 513–525. [Google Scholar] [CrossRef]
- Bligård, L.O.; Strömberg, H.; Karlsson, M.A. Developers as Users: Exploring the Experiences of Using a New Theoretical Method for Usability Assessment. Adv. Hum. Comput. Interact. 2017. [Google Scholar] [CrossRef] [Green Version]
- Schmettow, M.; Schnittker, R.; Schraagen, J.M. An extended protocol for usability validation of medical devices: Research design and reference model. J. Biomed. Inform. 2017, 69, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Norris, B.J.; Murphy, E.; Crowe, J.A. Medical device development: The challenge for ergonomics. Appl. Ergon. 2008, 39, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.L.; Barnett, J. Integrating the results of user research into medical device development: Insights from a case study. BMC Med. Inform. Decis. Mak. 2012, 12, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 14155. Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice; International Standard Organization: Geneva, Switzerland, 2011. [Google Scholar]
- ISO 14971. Medical Devices—Application of Risk Management to Medical Devices; International Standard Organization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 62366-2. Medical Devices—Part 2: Guidance on the Application of Usability Engineering to Medical Devices; International Standard Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An empirical evaluation of the system usability scale. Int. J. Hum. Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Martins, A.I.; Rosa, A.F.; Queirós, A.; Silva, A.; Rocha, N.P. European Portuguese validation of the System Usability Scale (SUS). Procedia Comput. Sci. 2015, 67, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.R. Psychometric Evaluation of the Post-Study System Usability Questionaire: The PSSUQ. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Atlanta, GA, USA, 12–16 October 1992; pp. 1259–1263. [Google Scholar]
- Lewis, J.R. Psychometric Evaluation of the PSSUQ Using Data from Five Years of Usability Studies. Int. J. Hum. Comput. Interact. 2002, 14, 463–488. [Google Scholar] [CrossRef]
- Rosa, A.F.; Martins, A.I.; Costa, V.; Queirós, A.; Silva, A.; Rocha, N.P. Validação para português europeu do Post-Study System Usability Questionnaire (PPSUQ). In Proceedings of the 10th Iberian Conference on Information Systems and Technologies (CISTI), Aveiro, Portugal, 17–20 June 2015. [Google Scholar]
- Martins, A.I.; Rosa, A.F.; Queirós, A.; Silva, A.; Rocha, N.P. Definition and validation of the ICF-Usability Scale. Procedia Comput. Sci. 2015, 67, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.I.; Queirós, A.; Rocha, N.P. Validation of a usability assessment instrument according to the evaluators’ perspetive about users’ performance. Univers. Access Inf. Soc. 2020, 19, 515–525. [Google Scholar] [CrossRef]
- Demers, L.; Weiss-Lambrou, R.; Ska, B. The Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST 2.0): An overview and recent progress. Technol. Disabil. 2019, 14, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.M. Measuring usability with the USE questionnaire. Usability Interface 2001, 8, 3–6. [Google Scholar]
- Lewis, J.R. Psychometric evaluation of an after-scenario questionnaire for computer usability studies: The ASQ. ACM SIGCHI Bulletin. 1991, 23, 78–81. [Google Scholar] [CrossRef]
- Lewis, J.R. Psychometric evaluation of an after-scenario questionnaire for computer usability studies. ACM SIGCHI Bull. 2007, 23, 78–81. [Google Scholar]
- Ghanbary Sartang, A.; Ashnagar, M.; Habibi, E.; Sadeghi, S. Evaluation of Rating Scale Mental Effort (RSME) effectiveness for mental workload assessment in nurses. J. Occup. Health Epidemiol. 2016, 5, 211–217. [Google Scholar] [CrossRef]
- Davis, F.D. Perceived Usefulness, Perceived Ease Of Use, And User Acceptance. MIS Q. 1989, 13, 319–339. [Google Scholar] [CrossRef] [Green Version]
- Davis, F.D.; Bagozzi, R.P.; Warshaw, P.R. User Acceptance of Computer Technology: A Comparison of Two Theoretical Models. Manag. Sci. 1989, 35. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, V. Determinants of perceived ease of use: Integrating control, intrinsic motivation, and emotion into the technology acceptance model. Inf. Syst. Res. 2000, 11, 342–365. [Google Scholar] [CrossRef] [Green Version]
- Santos, I.C.T.; Tavares, J.M.R.S. Additional peculiarities of medical devices that should be considered in their development process. Expert Rev. Med. Devices 2013, 10, 411–420. [Google Scholar] [CrossRef]
- Bardin, L. L’analyse de Contenu; Quadrige/PUF: Paris, France, 2007. [Google Scholar]
- Yen, P.Y.; Walker, D.M.; Smith, J.M.G.; Zhou, M.P.; Menser, T.L.; McAlearney, A.S. Usability evaluation of a commercial inpatient portal. Int. J. Med. Inf. 2018, 110, 10–18. [Google Scholar] [CrossRef]
- Parreira, P.; Sousa, L.B.; Marques, I.A.; Costa, P.; Braga, L.; Cruz, A.; Salgueiro-Oliveira, A. Double-chamber syringe versus classic syringes for peripheral intravenous drug administration and catheter flushing: A study protocol for a randomised controlled trial. Trials 2020, 21, 78. [Google Scholar] [CrossRef] [Green Version]
- Krell, M. Evaluating an instrument to measure mental load and mental effort using Item Response Theory. Sci. Educ. Rev. Lett. 2015, 3, 1–6. [Google Scholar]
- Krell, M. Evaluating an instrument to measure mental load and mental effort considering different sources of validity evidence. Cogent Educ. 2017, 4, 1–10. [Google Scholar] [CrossRef]
| Concept Stage (n = 16) | Semi-Functional Prototype Stage (n = 22) | Functional Prototype Stage (n = 30) | |
|---|---|---|---|
| Sex n (%) | |||
| Male | 5 (31.3%) | 7 (31.8%) | 8 (26.7%) |
| Female | 11 (68.7%) | 15 (68.2%) | 22 (73.3%) |
| Age (years) | 39.25 ± 10.096 | 37.86 ± 9.083 | 36.57 ± 8.012 |
| M ± SD (Min.–Max.) | 25–55 | 25–55 | 26–55 |
| Education n (%) | |||
| Bachelors’ degree | 3 (18.7%) | 3 (13.6%) | 17 (56.7%) |
| Post-graduate/Specialty | 4 (25.0%) | 6 (27.3%) | 4 (13.3%) |
| Master’s degree | 9 (56.3%) | 12 (54.5%) | 7 (23.3%) |
| Ph.D. | - | 1 (4.5%) | 2 (6.7%) |
| Professional time (months) | 195.56 ± 120.434 | 185.32 ± 106.512 | 163.87 ± 97.604 |
| M ± SD (Min.–Max.) | 25–55 | 36–372 | 24–384 |
| Department n (%) | |||
| Operating room | 5 (31.3%) | 6 (27.3%) | 2 (6.7%) |
| General hospital | 1 (6.2%) | 1 (4.5%) | 5 (16.7%) |
| Internal medicine | - | 3 (13.6%) | 3 (10.0%) |
| Research unit | 3 (18.8%) | 3 (13.6%) | 2 (6.7%) |
| Cancer unit | 1 (6.2%) | 2 (9.1%) | 2 (6.7%) |
| Gastroenterology | - | - | 2 (6.7%) |
| Emergency Room | - | - | 3 (10.0%) |
| Orthopaedics | 2 (12.5%) | 2 (9.1%) | 1 (3.3%) |
| Rheumatology | - | - | 2 (6.7%) |
| Intensive care unit | 2 (12.5%) | 2 (9.1%) | - |
| Physical medicine/Rehabilitation | - | - | 2 (6.7%) |
| Pneumology | 1 (6.2%) | 1 (4.5%) | - |
| Haematology | - | - | 1 (3.3%) |
| Pain consultation | - | 1 (4.5%) | - |
| Psychiatric ward | - | - | 1 (3.3%) |
| Burn unit | 1 (6.2%) | 1 (4.5%) | - |
| Urology | - | - | 1 (3.3%) |
| Continued care unit | - | - | 1 (3.3%) |
| Unemployed | - | - | 1 (3.3%) |
| Type of healthcare institution n (%) | |||
| Public institutions | 13 (81.3%) | 19 (86.4%) | 25 (83.3%) |
| Private institutions | - | - | 2 (6.7%) |
| Other (Teaching/Research) | 3 (18.7%) | 3 (13.6%) | 3 (10.0%) |
| Time at the current professional unit (months) | 135.25 ± 124.509 | 124.55 ± 110.403 | 185.32 ± 106.512 |
| M ± SD (Min.–Max.) | 6–372 | 6–372 | 1–384 |
| Items | Item-Total Correlation | α (If Item Excluded) | Item-Domain Correlation |
|---|---|---|---|
| 1. is useful for my work. | 0.645 ** | 0.975 | 0.771 ** |
| 2. facilitates the performance of my tasks. | 0.751 ** | 0.975 | 0.890 ** |
| 3. helps me to be more effective. | 0.754 ** | 0.975 | 0.901 ** |
| 4. helps me to be more efficient. | 0.781 ** | 0.975 | 0.897 ** |
| 5. achieves everything I would expect it to do. | 0.763 ** | 0.975 | 0.803 ** |
| 6. allows me to complete my tasks. | 0.728 ** | 0.975 | 0.767 ** |
| 7. allows me to complete my tasks easily. | 0.763 ** | 0.975 | 0.814 ** |
| 8. allows me to complete my tasks quickly. | 0.717 ** | 0.975 | 0.797 ** |
| 9. allows me to have better control over my tasks. | 0.725 ** | 0.975 | 0.835 ** |
| 10. helps me to be more productive. | 0.777 ** | 0.975 | 0.844 ** |
| 11. allows me to provide safer care. | 0.661 ** | 0.975 | 0.751 ** |
| 12. answers my needs. | 0.837 ** | 0.975 | 0.860 ** |
| Items | Item-Total Correlation | α (If Item Excluded) | Item-Domain Correlation |
|---|---|---|---|
| 13. is easy to use. | 0.795 ** | 0.975 | 0.861 ** |
| 14. is simple to use. | 0.815 ** | 0.975 | 0.870 ** |
| 15. is user-friendly. | 0.721 ** | 0.975 | 0.802 ** |
| 16. requires few steps to accomplish my work. | 0.670 ** | 0.975 | 0.815 ** |
| 17. is flexible to use according to my needs. | 0.701 ** | 0.975 | 0.663 ** |
| 18. does not require physical effort to use it. | 0.761 ** | 0.975 | 0.837 ** |
| 19. does not require mental effort to use it. | 0.509 ** | 0.975 | 0.653 ** |
| 20. allows me to complete tasks in a logical sequence. | 0.573 ** | 0.975 | 0.702 ** |
| 21. is not associated with significant possibilities of error in its use. | 0.651 ** | 0.975 | 0.717 ** |
| 22. allows me to recover from mistakes quickly and easily. | 0.651 ** | 0.975 | 0.642 ** |
| Items | Item-Total Correlation | α (If Item Excluded) | Item-Domain Correlation |
|---|---|---|---|
| 23. I learned to use it quickly. | 0.633 ** | 0.975 | 0.888 ** |
| 24. I learned to use it easily. | 0.740 ** | 0.975 | 0.853 ** |
| 25. I easily remember how to use it. | 0.611 ** | 0.975 | 0.867 ** |
| 26. I quickly became skilful with it. | 0.651 ** | 0.975 | 0.863 ** |
| 27. it is not necessary too much previous knowledge to use it. | 0.490 ** | 0.976 | 0.766 ** |
| 28. there is no need for written instructions to use it. | 0.335 * | 0.977 | 0.705 ** |
| Items | Item-Total Correlation | α (If Item Excluded) | Item-Domain Correlation |
|---|---|---|---|
| 29. I will be satisfied with it. | 0.898 ** | 0.974 | 0.917 ** |
| 30. I would recommend it to colleagues. | 0.884 ** | 0.974 | 0.914 ** |
| 31. it will allow the performance of my tasks. | 0.875 ** | 0.974 | 0.902 ** |
| 32. it will be interesting for the performance of my tasks. | 0.867 ** | 0.974 | 0.915 ** |
| 33. I feel I need to have it in my work. | 0.608 ** | 0.974 | 0.656 ** |
| 34. it will be pleasant to use. | 0.810 ** | 0.975 | 0.823 ** |
| 35. I will feel comfortable in using it. | 0.851 ** | 0.975 | 0.861 ** |
| 36. I will feel confident in using it. | 0.821 ** | 0.975 | 0.863 ** |
| 37. I will feel secure in using it. | 0.788 ** | 0.975 | 0.835 ** |
| 38. the dimensions of the device are adjusted. | 0.804 ** | 0.975 | 0.814 ** |
| 39. the weight of the device is adjusted. | 0.479 ** | 0.976 | 0.577 ** |
| 40. the appearance of the device is adjusted. | 0.531 ** | 0.976 | 0.560 ** |
| 41. I will like to use it frequently. | 0.798 ** | 0.975 | 0.893 ** |
| 42. it will be easy to adjust it during the performance of my work. | 0.725 ** | 0.975 | 0.802 ** |
| Usability Dimensions | Concept (n = 16) | r | Semi-Functional Prototype (n = 22) | r | Functional Prototype (n = 30) | r | |
|---|---|---|---|---|---|---|---|
| Global score | M ± SD | 5.80 ± 0.534 | −0.174 | 5.80 ± 0.786 | −0.250 | 5.28 ± 0.944 | 0.273 |
| Min.–Max. | 4.81–6.76 | 3.93–7.00 | 3.19–7.00 | ||||
| Usefulness | M ± SD | 6.11 ± 0.593 | 0.062 | 5.95 ± 0.875 | −0.283 | 5.36 ± 1.160 | 0.309 |
| Min.–Max. | 4.75–7.00 | 3.58–7.00 | 2.00–7.00 | ||||
| Ease of use | M ± SD | 5.69 ± 0.597 | 0.267 | 5.72 ± 0.928 | 0.082 | 5.06 ± 1.013 | 0.183 |
| Min.–Max. | 4.30–6.40 | 3.20–7.00 | 3.20–7.00 | ||||
| Ease of learning | M ± SD | 6.05 ± 0.767 | −0.306 | 5.96 ± 0.916 | 0.111 | 5.53 ± 1.022 | 0.130 |
| Min.–Max. | 4.33–7.00 | 4.00–7.00 | 2.83–7.00 | ||||
| Satisfaction/Use intention | M ± SD | 5.93 ± 0.860 | −0.201 | 5.83 ± 0.826 | −0.070 | 5.37 ± 1.082 | 0.274 |
| Min.–Max. | 4.71–7.00 | 3.57–7.00 | 3.07–7.00 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parreira, P.; Sousa, L.B.; Marques, I.A.; Santos-Costa, P.; Cortez, S.; Carneiro, F.; Cruz, A.; Salgueiro-Oliveira, A. Usability Assessment of an Innovative Device in Infusion Therapy: A Mix-Method Approach Study. Int. J. Environ. Res. Public Health 2020, 17, 8335. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228335
Parreira P, Sousa LB, Marques IA, Santos-Costa P, Cortez S, Carneiro F, Cruz A, Salgueiro-Oliveira A. Usability Assessment of an Innovative Device in Infusion Therapy: A Mix-Method Approach Study. International Journal of Environmental Research and Public Health. 2020; 17(22):8335. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228335
Chicago/Turabian StyleParreira, Pedro, Liliana B. Sousa, Inês A. Marques, Paulo Santos-Costa, Sara Cortez, Filipa Carneiro, Arménio Cruz, and Anabela Salgueiro-Oliveira. 2020. "Usability Assessment of an Innovative Device in Infusion Therapy: A Mix-Method Approach Study" International Journal of Environmental Research and Public Health 17, no. 22: 8335. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17228335








